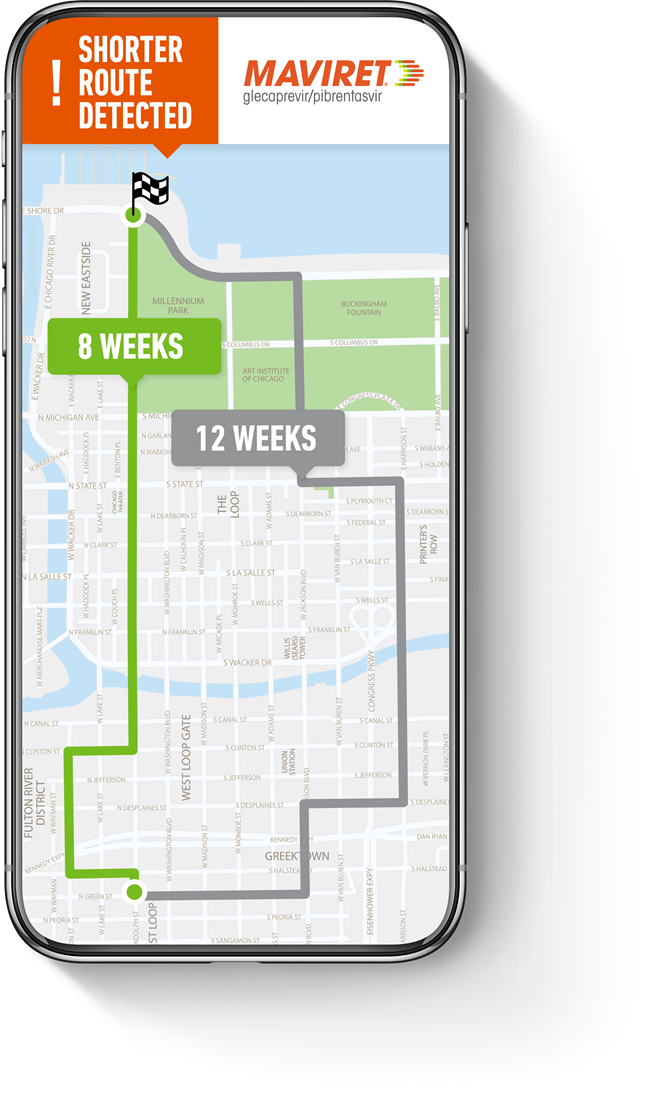
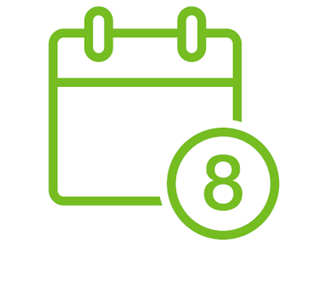
8 WEEKS: THE SHORTEST ROUTE TO <CURE*>
MAVIRET offers a pangenotypic 8-week duration for treatment-naïve HCV patients1†
< INSERT LOCAL SAFETY >
†Refers to GT 1–6, excluding decompensated cirrhotic patients and liver or kidney transplant recipients. MAVIRET is not indicated in decompensated cirrhosis. The recommended duration of MAVIRET is 12 weeks in liver or kidney transplant recipients, with or without cirrhosis.
‡Tablets should be swallowed whole with food and not chewed, crushed, or broken.
<cure*> = sustained virologic response (SVR12), defined as HCV RNA less than the lower limit of quantification at 12 weeks after the end of treatment and was the primary endpoint in all the studies.1
Please see Safety Summary and full Prescribing Information
I want to receive more information via a product specialist
References
- MAVIRET [SmPC]. Ludwigshafen, Germany: AbbVie Deutschland GmbH & Co. KG;
- Zukerman E, Gutierrez J, Dylla D, et al. Integrated efficacy and safety analysis of GT1-6 treatment-naïve, non-cirrhotic and compensated cirrhotic patients who received 8 weeks of glecaprevir/pibrentasvir. Abstract presented at: 2020 European Association for the Study of the Liver (EASL) International Liver Congress. Abstract THU406.
ALL-MAVI-200050
Adverse events should be reported. Reporting forms and information can be found at yellowcard.mhra.gov.uk.
Adverse events should also be reported to AbbVie on uk_pvvendor@abbvie.com