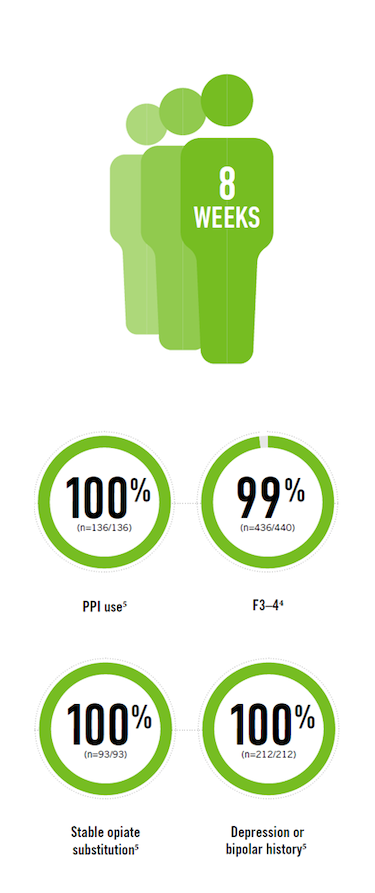
MAVIRET efficacy in treatment-naïve patients
High <cure*> rates (SVR12) in 8 weeks across genotypes1,4†
< INSERT LOCAL SAFETY >
†Refers to GT 1–6, excluding decompensated cirrhotic patients and liver or kidney transplant recipients. MAVIRET is not indicated in decompensated cirrhosis. The recommended duration of MAVIRET is 12 weeks in liver or kidney transplant recipients, with or without cirrhosis.
‡Data include non-cirrhotic, treatment-experienced patients to peginterferon, ribavirin, and/or sofosbuvir with genotypes 1, 2, 4, 5, and 6 infection. GT 3 patients included in this analysis were treatment-naïve only.
ITT = intention-to-treat; PP = per protocol data of ITT population excluding patients who experienced virological breakthrough, discontinued treatment prior to week 8, or had missing SVR12 data; RBV = ribavirin; TN = treatment-naïve; TN-CC = treatment-naïve, compensated cirrhotic; TN-NC = treatment-naïve, non-cirrhotic, TN-CC = treatment-naïve, compensated-cirrhotic.
<cure*> = sustained virologic response (SVR12), defined as HCV RNA less than the lower limit of quantification at 12 weeks after the end of treatment and was the primary endpoint in all the studies.1
TN data were pooled from 8-week arms of the ENDURANCE 1, ENDURANCE 3, SURVEYOR 1, SURVEYOR 2, EXPEDITION 2, and EXPEDITION 8 studies.1
TN-NC data were pooled from 8-week arms of the ENDURANCE 1, ENDURANCE 3, SURVEYOR 1, SURVEYOR 2, and EXPEDITION 2 studies.1
TN-CC data were from EXPEDITION 8 study.2
< INSERT LOCAL SAFETY >
†Refers to GT 1–6, excluding decompensated cirrhotic patients and liver or kidney transplant recipients. MAVIRET is not indicated in decompensated cirrhosis. The recommended duration of MAVIRET is 12 weeks in liver or kidney transplant recipients, with or without cirrhosis.
F3–4 = Stage 3 or Stage 4 liver fibrosis; mITT = ITT population modified to exclude subjects who did not achieve SVR12 for reasons other than virologic failure; PPI = proton pump-inhibitor.
<cure*>=sustained virologic response (SVR12), defined as HCV RNA less than the lower limit of quantification at 12 weeks after the end of treatment and was the primary endpoint in all the studies.1
Data were pooled from 8-week arms of the ENDURANCE 1, ENDURANCE 3, SURVEYOR 1, SURVEYOR 2, EXPEDITION 2, and EXPEDITION 8 studies.1
< INSERT LOCAL SAFETY >
†Refers to GT 1–6. MAVIRET is not indicated in decompensated cirrhosis. The recommended duration of MAVIRET is 12 weeks in liver or kidney transplant recipients, with or without cirrhosis.
‡Includes patients who reached SVR12 visit.
§Modified ITT (mITT)=ITT population modified to exclude patients who did not achieve SVR12 for reasons other than virologic failure.
<cure*> = sustained virologic response (SVR12), defined as HCV RNA less than the lower limit of quantification at 12 weeks after the end of treatment and was the primary endpoint in all the studies.1
Adverse event data6
- In the Safety Population (n=969) the most common adverse events (≥5% prevalence) were fatigue (10%) and headache (7%)
- Three patients experienced a serious adverse event related or possibly related to therapy (<1%)
- Three patients experienced an adverse event that led to discontinuation (<1%)
- No patients experienced ALT elevations grade >5 x ULN; <1% had AST elevations grade >5 x ULN; and <1% had total bilirubin elevations grade >3 x ULN
Financial support of analysis and publication was provided by AbbVie.
Real-world evidence is collected outside of controlled clinical trials and has inherent limitations including a lesser ability to control for confounding factors.
ULN=upper limit of normal.
NOTE TO AFFILIATE:
A. Evaluate the use of 'NOW APPROVED.' Please refer to local regulatory guidelines for direction.
B. Replace "cure" or "cured" with "SVR12" when locally appropriate.
C. GPS artwork of a longer 8-week route shown here is available, when local regulations do not allow depiction of a straight route. See the HCV Global Brand Book for further information.
Please see Safety Summary and full Prescribing Information
I want to receive more information via a product specialist
References
- MAVIRET [SmPC]. Ludwigshafen, Germany: AbbVie Deutschland GmbH & Co. KG; <current>.
- Brown RS, Buti M, Rodrigues L, et al. Glecaprevir/pibrentasvir for 8 weeks in treatment-naïve patients with chronic HCV genotypes 1–6 and compensated cirrhosis: the EXPEDITION-8 trial. J Hepatol. 2020;72(3);441-449. doi:10.1016/j.jhep.2019.10.020
- Puoti M, Foster GR, Wang S, et al. High SVR12 with 8-week and 12-week glecaprevir/pibrentasvir therapy: an integrated analysis of HCV genotype 1–6 patients without cirrhosis. J Hepatol. 2018;69(2):293-300. doi:10.1016/j.jhep.2018.03.007
- Zukerman E, Gutierrez J, Dylla D, et al. Integrated efficacy and safety analysis of GT1-6 treatment-naïve, non-cirrhotic and compensated cirrhotic patients who received 8 weeks of glecaprevir/pibrentasvir. Abstract presented at: 2020 European Association for the Study of the Liver (EASL) International Liver Congress. Abstract THU406.
- Data on file, AbbVie Inc. ABVRRTI69094.
- Wiegand J, Naumann U, Stoehr A, et al. Glecaprevir/pibrentasvir for the treatment of patients with chronic hepatitis C virus infection: updated real-world data from the German Hepatitis C-Registry. Poster presented at: 69th Annual Meeting of the American Association for the Study of Liver Diseases (AASLD); November 9–13, 2018. San Francisco, California.
Adverse events should be reported. Reporting forms and information can be found at yellowcard.mhra.gov.uk.
Adverse events should also be reported to AbbVie on uk_pvvendor@abbvie.com