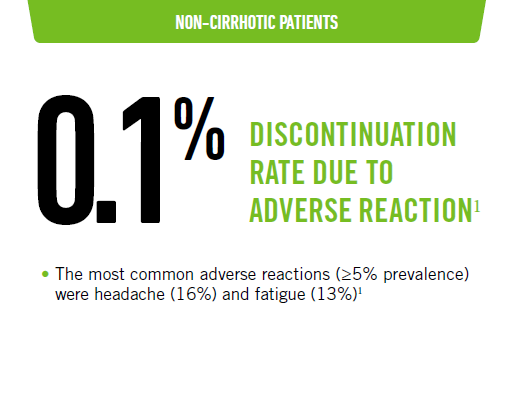
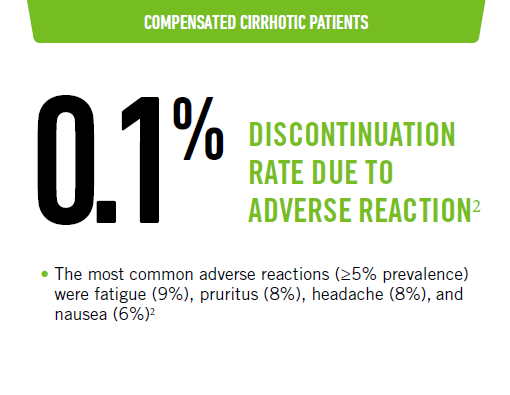
MAVIRET tolerability profile - treatment naïve
Favorable tolerability profile: 8-week treatment duration1,2
- Most adverse reactions were mild in severity
- The type and severity of adverse reactions in subjects with cirrhosis were comparable overall to those seen in subjects without cirrhosis
TN-NC data were pooled from 8-week arms of the ENDURANCE 1, ENDURANCE 3, SURVEYOR 1, SURVEYOR 2, and EXPEDITION 2 studies.1
TN-CC data were from EXPEDITION 8 study.2
I want to receive more information via a product specialist
References
- Puoti M, Foster GR, Wang S, et al. High SVR12 with 8-week and 12-week glecaprevir/pibrentasvir therapy: an integrated analysis of HCV genotype 1–6 patients without cirrhosis. J Hepatol. 2018;69(2):293-300. doi:10.1016/j.jhep.2018.03.007
- Brown RS, Buti M, Rodrigues L, et al. Glecaprevir/pibrentasvir for 8 weeks in treatment-naïve patients with chronic HCV genotypes 1–6 and compensated cirrhosis: the EXPEDITION-8 trial. J Hepatol. Published online November 2, 2019. doi:10.1016/j.jhep.2019.10.020
Adverse events should be reported. Reporting forms and information can be found at yellowcard.mhra.gov.uk.
Adverse events should also be reported to AbbVie on uk_pvvendor@abbvie.com