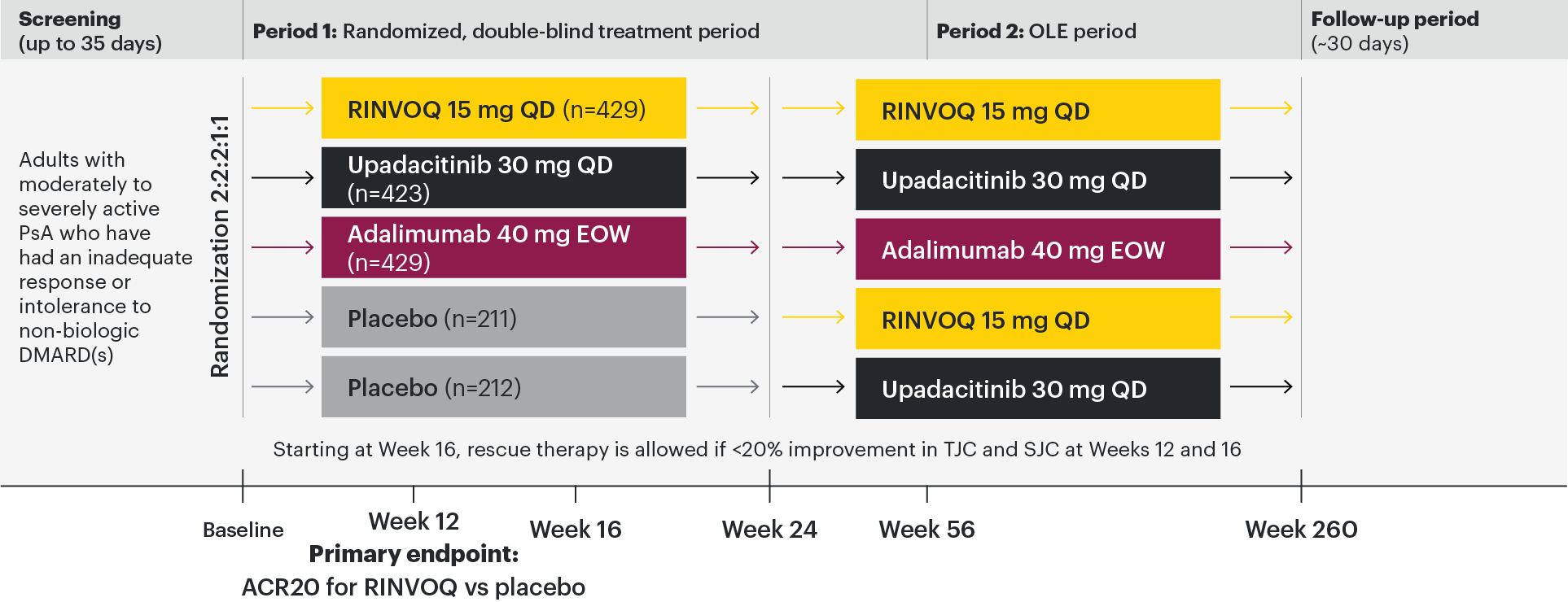
A Phase 3 study and open-label extension investigating the efficacy and safety of RINVOQ compared with placebo and adalimumab for the treatment of active PsA in patients with an inadequate response to non-biologic DMARDs1,2
The approved dose of RINVOQ in active PsA is 15 mg once daily. Upadacitinib 30 mg QD is not an approved dose in PsA.
Stable treatment of NSAIDs, corticosteroids, and ≤2 non-biologic DMARDs were permitted, but not required.
All subjects received x-rays of hands and feet at screening, Week 24, Week 56, Week 104, and Week 152. At Week 16, rescue therapy was offered to subjects classified as nonresponders (defined as not achieving at least 20% improvement in tender joint count and swollen joint count at both Week 12 and Week 16). At Week 24, all placebo subjects were switched to RINVOQ 15 mg QD or upadacitinib 30 mg QD (1:1 ratio) regardless of response.
Primary
ACR20 response for RINVOQ vs placebo at Week 12
Key ranked secondary endpoints
(RINVOQ vs placebo unless noted)
- Change from baseline in HAQ-DI at Week 12
- Proportion of subjects achieving a slGA of Psoriasis of 0 or 1 and at least a 2-point improvement from baseline at Week 16
- PASI 75 response at Week 16
- Change from baseline mTSS at Week 24
- Proportion of subjects achieving MDA at Week 24
- Proportion of subjects with resolution of enthesitis (LEI=0) at Week 24
- ACR20 response rate at Week 12 (noninferiority vs adalimumab)
- Change from baseline in SF-36 PCS at Week 12
- Change from baseline in FACIT-F at Week 12
- ACR20 response rate at Week 12 (superiority vs adalimumab)*
- Proportion of subjects with resolution of dactylitis (LDI=0) at Week 24†
- Change from baseline in patient's assessment of pain NRS at Week 12 (superiority vs adalimumab)†
- Change from baseline in HAQ-DI at Week 12 (superiority vs adalimumab); and†
- Change from baseline in SAPS at Week 16†
Other key secondary endpoints
(RINVOQ vs placebo)
- ACR50/70 at Week 12†
- ACR20 at Week 2†
Safety assessments
Data for treatment-emergent adverse events and laboratory assessments were collected during the study. Treatment-emergent adverse events were defined as adverse events that began or worsened in severity after the first dose of study medication through 30 days after the last dose.
*Superiority of RINVOQ 15 mg vs adalimumab could not be demonstrated, which prevented the testing of significance for secondary endpoints lower in the testing hierarchy.
†Not tested for significance / not multiplicity-controlled; no clinical inferences can be drawn from these data.
- ≥18 years old at screening
- Clinical diagnosis of PsA with symptom onset ≥6 months prior to screening and fulfillment of the CASPAR criteria
- Active disease at baseline defined as ≥3 tender joints and ≥3 swollen joints
- Presence at screening of either ≥1 erosion on x-ray as determined by central imaging review or hs-CRP >ULN
- Diagnosis of active plaque psoriasis or documented history of plaque psoriasis
- Inadequate response or intolerance to treatment with at least one non-biologic DMARD*
- On ≤2 non-biologic DMARDs
*Lack of efficacy after ≥12 weeks of therapy; intolerance or contraindication as defined by investigator.
- Prior exposure to any JAK inhibitor
- Prior exposure to any bDMARD
At baseline, 1,393 (82%) of patients were on at least 1 concomitant non-biologic DMARD; 1,084 (64%) of patients received concomitant MTX, only; and 311 (18%) of patients were on monotherapy.
ACR20: improvement of at least 20% in the American College of Rheumatology core criteria; bDMARD: biological disease-modifying antirheumatic drug; CASPAR: Classification Criteria for Psoriatic Arthritis; DMARD: disease-modifying antirheumatic drug; EOW: every other week; hs-CRP: high-sensitivity C-reactive protein; JAK: Janus kinase; MTX: methotrexate; OLE: open-label extension; QD: once daily; SJC: swollen joint count; TJC: tender joint count; ULN: upper limit of normal.
In patients with active PsA and an inadequate response to non-biologic DMARDs
RINVOQ joint symptom improvement was superior to placebo for ACR20 at Week 12
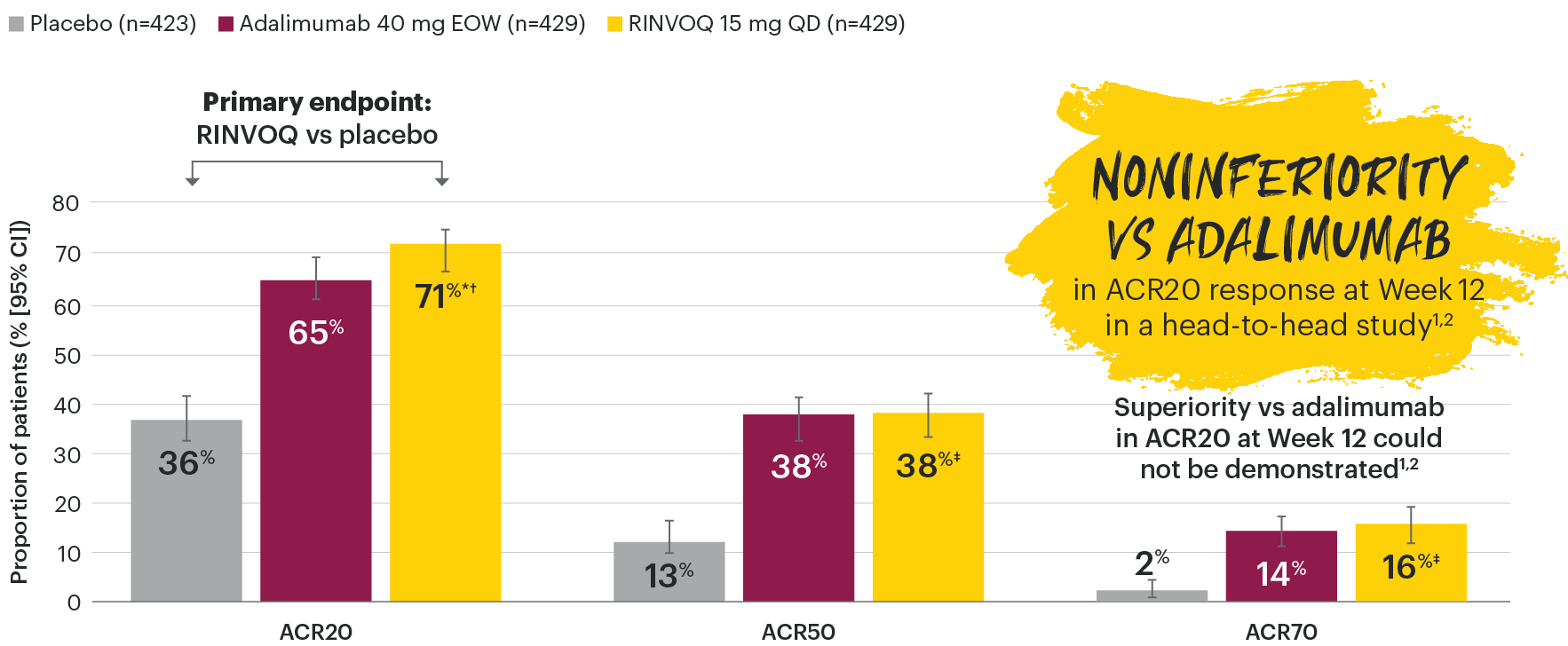
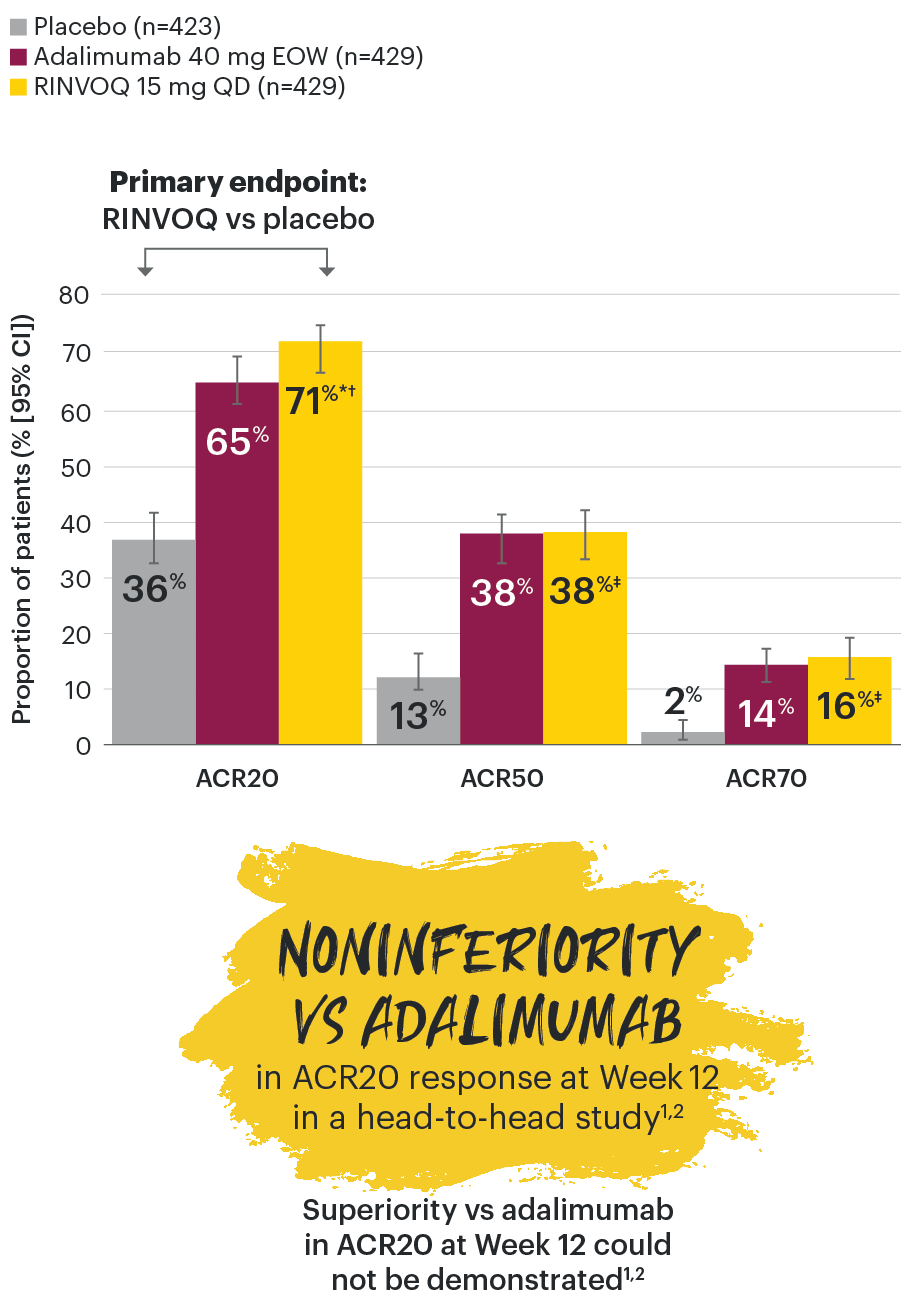
SELECT-PsA 1: ACR20/50/70 response rates at Week 12 (NRI)1-3
NOTE TO AFFILIATES: Please evaluate y-axis scale throughout, per local regulations.
*P≤0.001 vs placebo, statistically significant in the multiplicity-controlled analysis.
†P≤0.001 vs adalimumab for noninferiority, statistically significant in the multiplicity-controlled analysis.
‡Nominal P≤0.001 vs placebo. No clinical inferences can be drawn.3
ACR20 for RINVOQ vs placebo at Week 12 was a primary multiplicity-controlled endpoint.
ACR20 noninferiority vs adalimumab at Week 12 was a key ranked secondary, multiplicity-controlled endpoint.
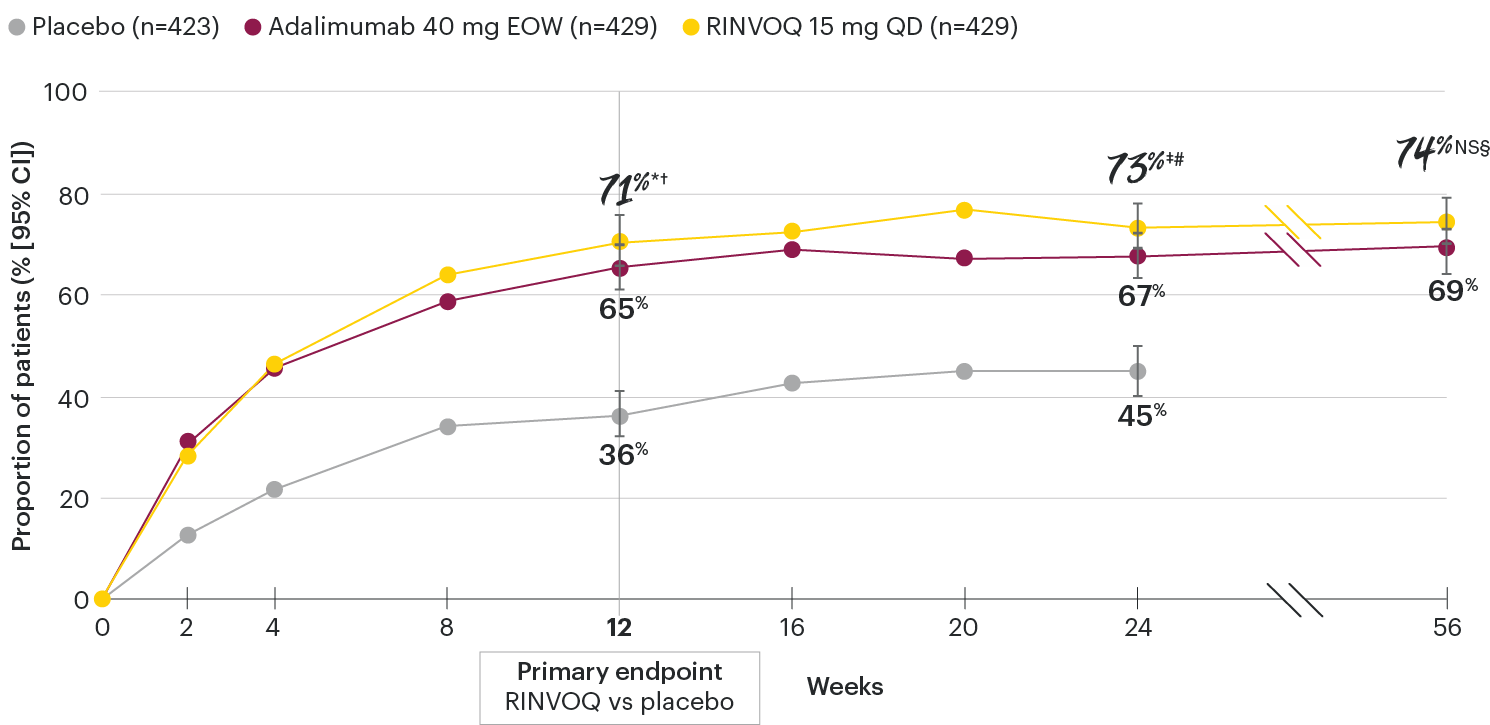
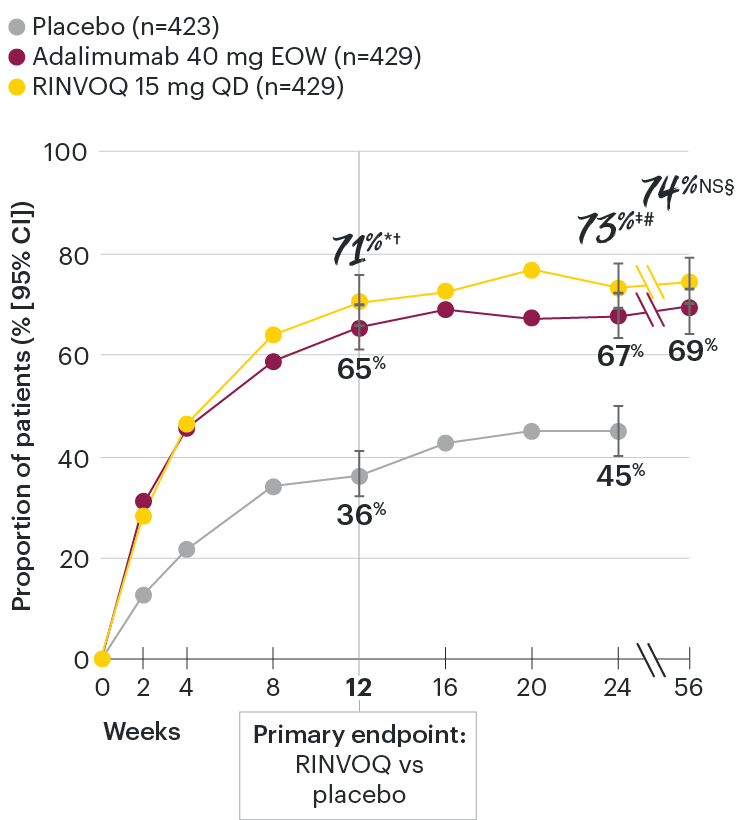
In patients with active PsA and an inadequate response to non-biologic DMARDs
ACR20 response rates over time
SELECT-PsA 1: ACR20 response rates through Week 56 (NRI)1-4
*P≤0.001 vs placebo, statistically significant in the multiplicity-controlled analysis.
†P≤0.001 vs adalimumab for noninferiority, statistically significant in the multiplicity-controlled analysis.
‡Nominal P≤0.001 vs placebo.3
#Nominal P≤0.05 vs adalimumab.3
NS§Not significant vs adalimumab.4
Nominal P-values denote data not controlled for multiplicity. No clinical inferences can be drawn.
Superiority vs adalimumab in ACR20 at Week 12 could not be demonstrated.2.
Treatment groups are by initial randomization. Data shown through Week 24 are from the Week 24 data cut of SELECT-PsA 1. Data at Week 56 are from the Week 56 data cut of SELECT-PsA 1 and may include differences when compared with the primary Week 24 analysis.
In patients with active PsA and an inadequate response to non-biologic DMARDs
ACR50 response rates over time
SELECT-PsA 1: ACR50 response rates through Week 56 (NRI)1-4
‡Nominal P≤0.001 vs placebo.3
#Nominal P≤0.05 vs adalimumab.3,4
Nominal P-values denote data not controlled for multiplicity. No clinical inferences can be drawn.
Treatment groups are by initial randomization. Data shown through Week 24 are from the Week 24 data cut of SELECT-PsA 1. Data at Week 56 are from the Week 56 data cut of SELECT-PsA 1 and may include differences when compared with the primary Week 24 analysis.
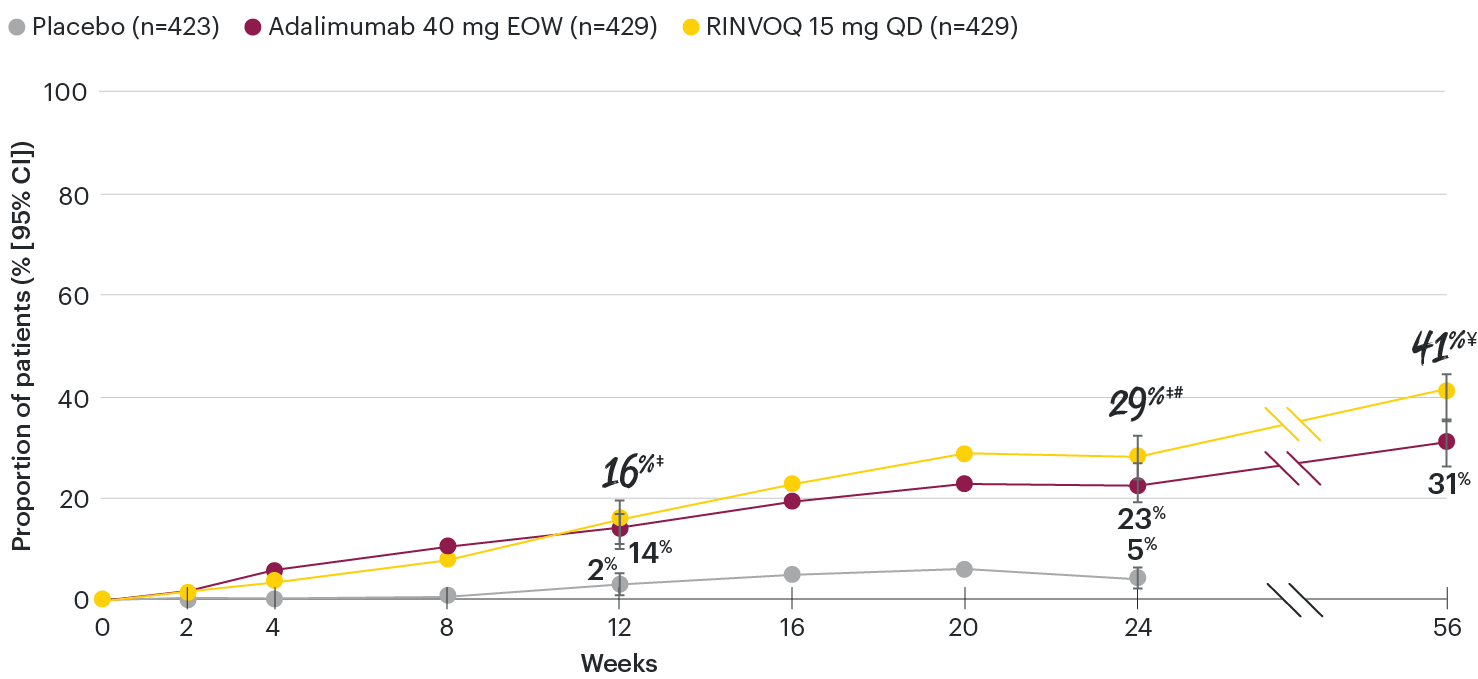
In patients with active PsA and an inadequate response to non-biologic DMARDs
ACR70 response rates over time
SELECT-PsA 1: ACR70 response rates through Week 56 (NRI)1-4
‡Nominal P≤0.001 vs placebo.3
#Nominal P≤0.05 vs adalimumab.3
¥Nominal P≤0.01 vs adalimumab.4
Nominal P-values denote data not controlled for multiplicity. No clinical inferences can be drawn.
Treatment groups are by initial randomization. Data shown through Week 24 are from the Week 24 data cut of SELECT-PsA 1. Data at Week 56 are from the Week 56 data cut of SELECT-PsA 1 and may include differences when compared with the primary Week 24 analysis.
DATA LIMITATIONS: Data not labeled as a primary or ranked secondary endpoint were prespecified, however they were not ranked or controlled for multiplicity; therefore, treatment differences could represent chance findings. No conclusions regarding these comparisons can be made.
95% confidence intervals are displayed as error bars in the chart. Missing data were handled using NRI.
ACR20/50/70: American College of Rheumatology 20%/50%/70% improvement in both tender and swollen joint counts, plus three of the following: patient assessments of pain, global disease activity and physical function, physician global assessment of disease activity and acute phase reactant (high sensitivity C-reactive protein); csDMARD: conventional synthetic disease-modifying antirheumatic drug; EOW: every other week; NRI: nonresponder imputation; QD: once daily.
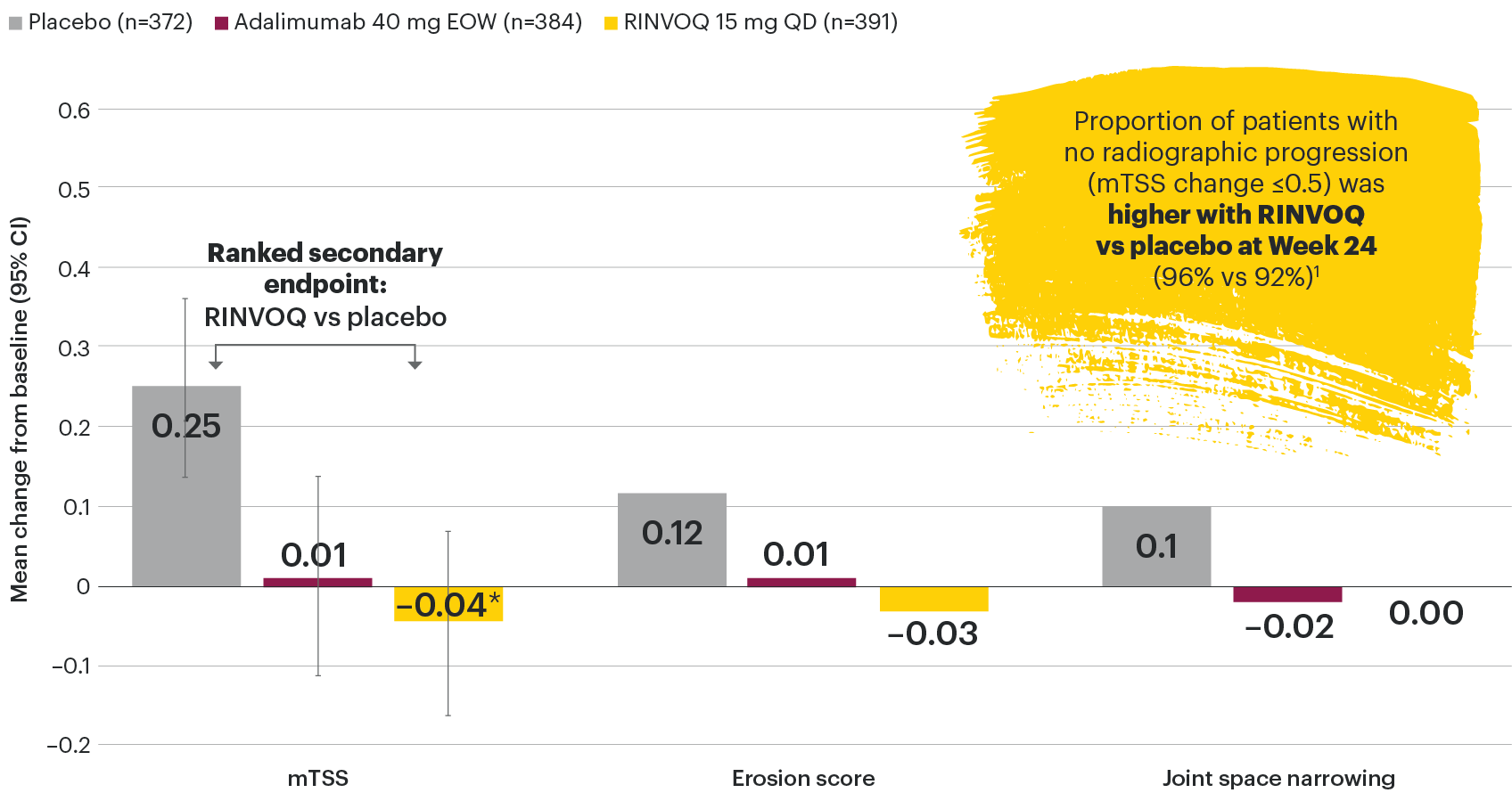
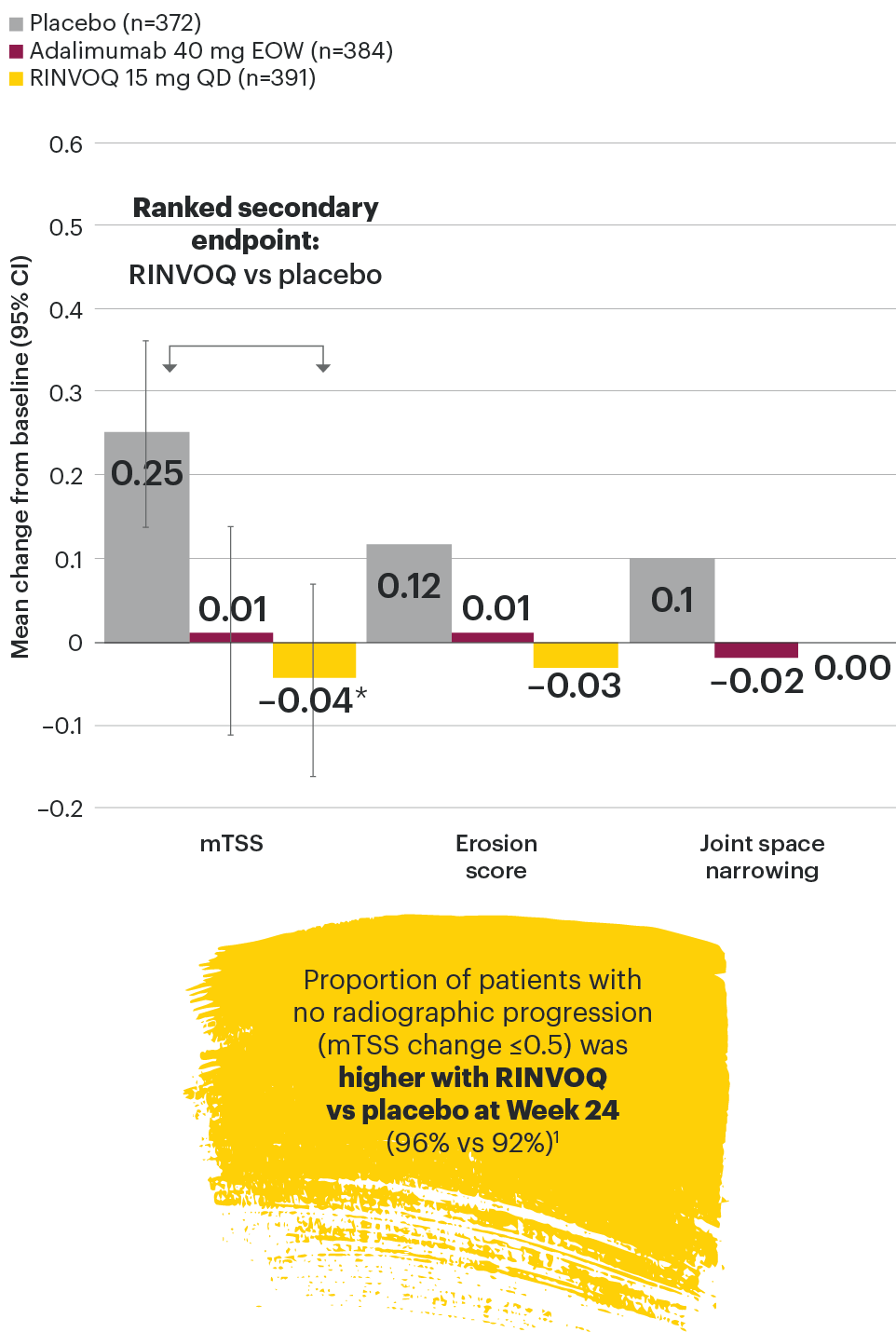
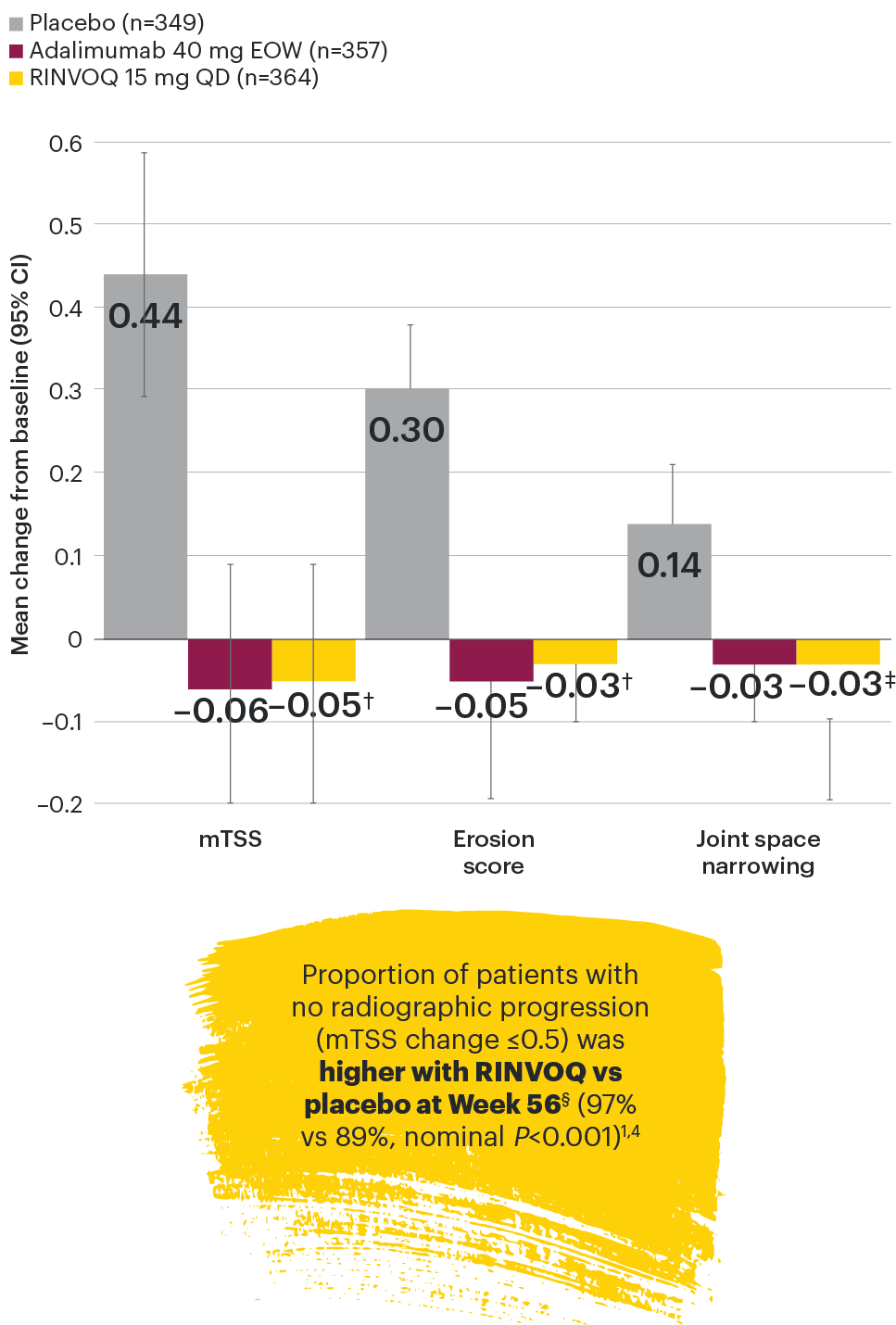
In patients with active PsA and an inadequate response to non-biologic DMARDs
Joint protection vs placebo across radiographic endpoints
SELECT-PsA 1: Inhibition of structural joint damage progression at Week 24 (linear extrapolation)1,2
DATA LIMITATIONS: Data not labeled as a primary or ranked secondary endpoint were prespecified, however they were not ranked or controlled for multiplicity; therefore, treatment differences could represent chance findings. No conclusions regarding these comparisons can be made. No statistical comparisons were made between RINVOQ and adalimumab groups for radiographic endpoints.
95% confidence intervals are displayed as error bars in the chart.
csDMARD: conventional synthetic disease-modifying antirheumatic drug; EOW: every other week; mTSS: modified total Sharp score; QD: once daily.
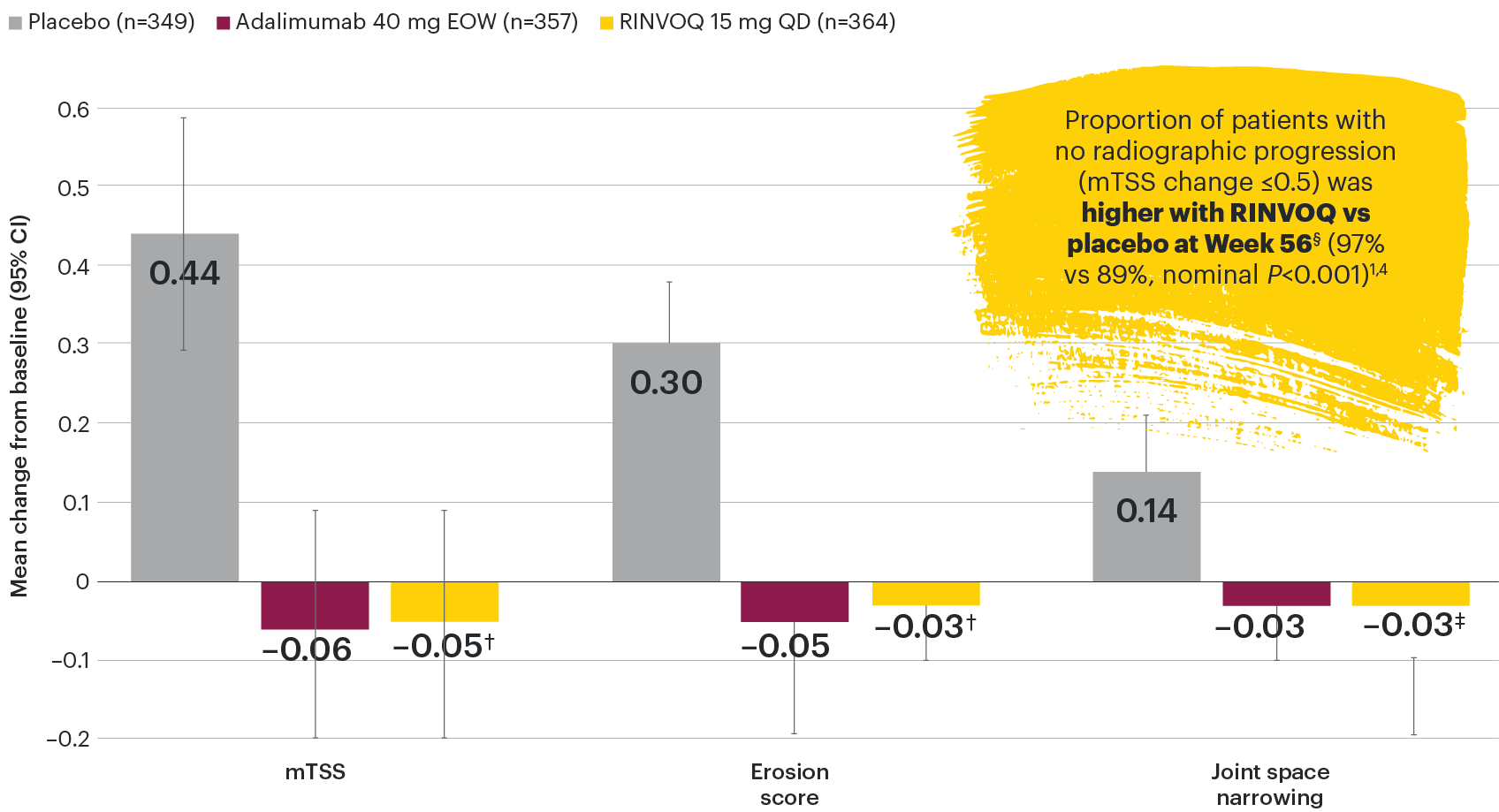
In patients with active PsA and an inadequate response to non-biologic DMARDs
Joint protection vs placebo across radiographic endpoints
SELECT-PsA 1: Inhibition of structural joint damage progression at Week 56 (linear extrapolation)1,4
†Nominal P<0.001 vs placebo.
‡Nominal P=0.005 vs placebo.
§All patients on placebo switched to RINVOQ at Week 24; all placebo data at Week 56 were derived using linear extrapolation. Nominal P-value denotes data not controlled for multiplicity. No clinical inferences can be drawn. Data at Week 56 are from the Week 56 data cut of SELECT-PsA 1 and may include differences when compared with the primary Week 24 analysis.
DATA LIMITATIONS: Data not labeled as a primary or ranked secondary endpoint were prespecified, however they were not ranked or controlled for multiplicity; therefore, treatment differences could represent chance findings. No conclusions regarding these comparisons can be made. No statistical comparisons were made between RINVOQ and adalimumab groups for radiographic endpoints.
95% confidence intervals are displayed as error bars in the chart.
csDMARD: conventional synthetic disease-modifying antirheumatic drug; EOW: every other week; mTSS: modified total Sharp score; QD: once daily.
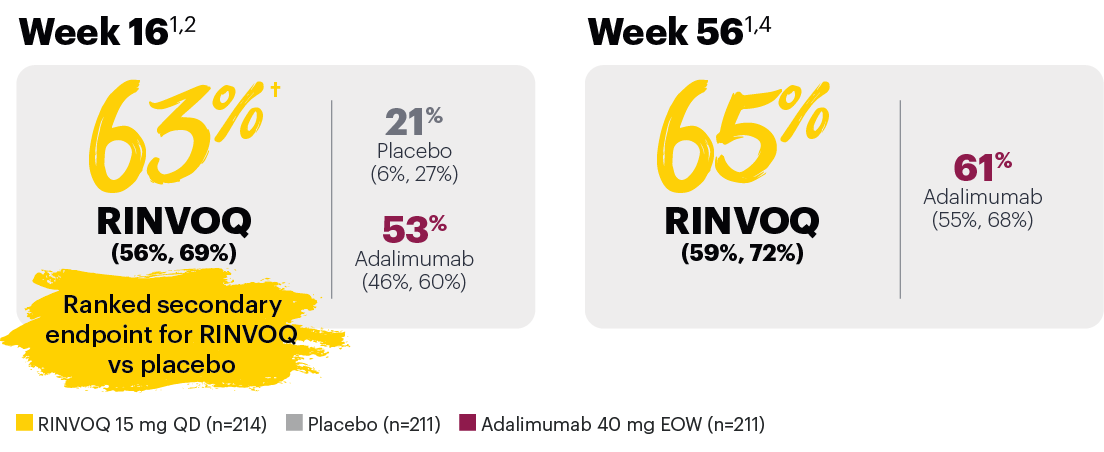
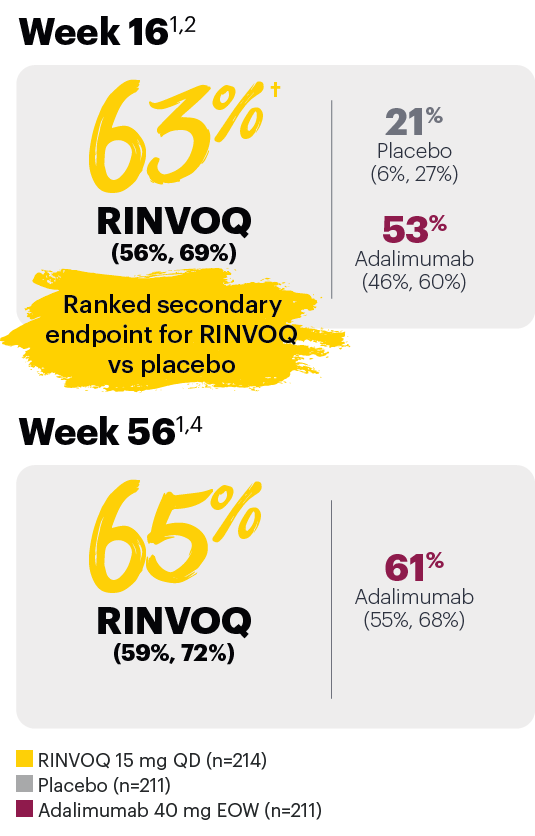
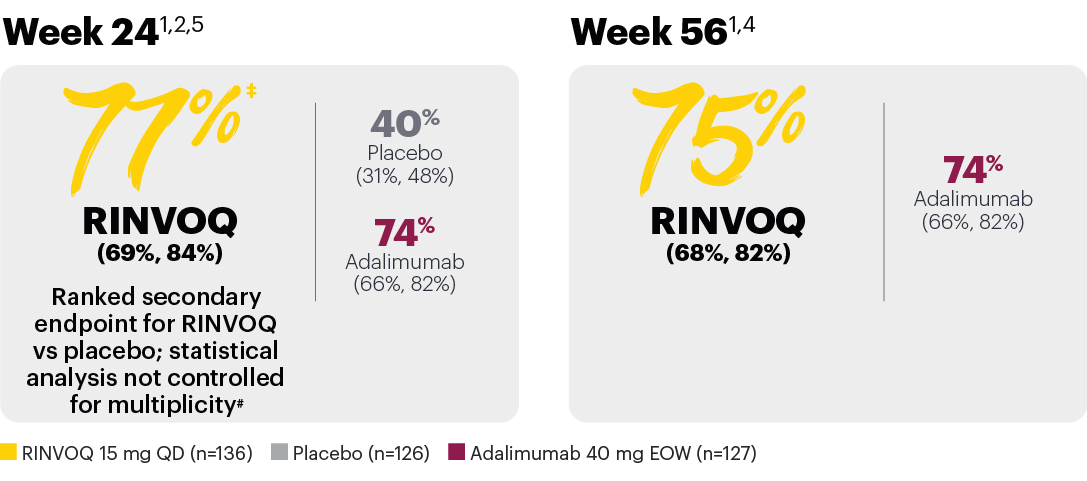
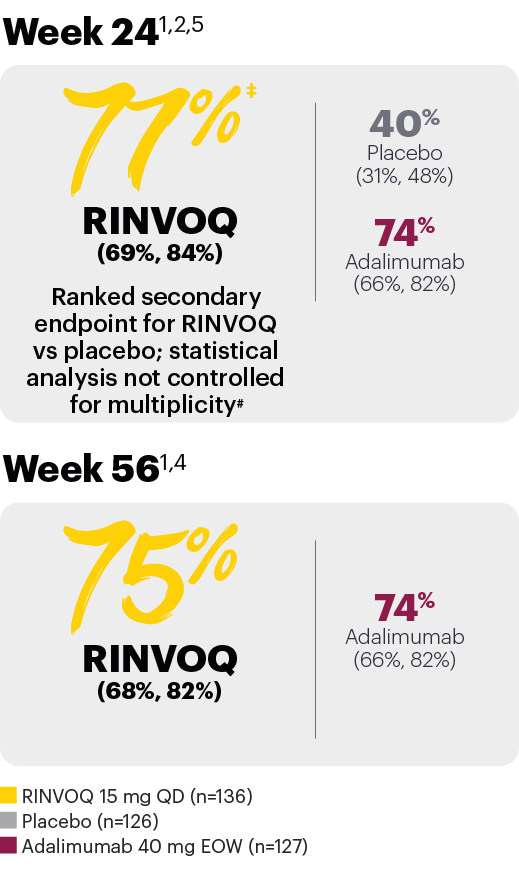
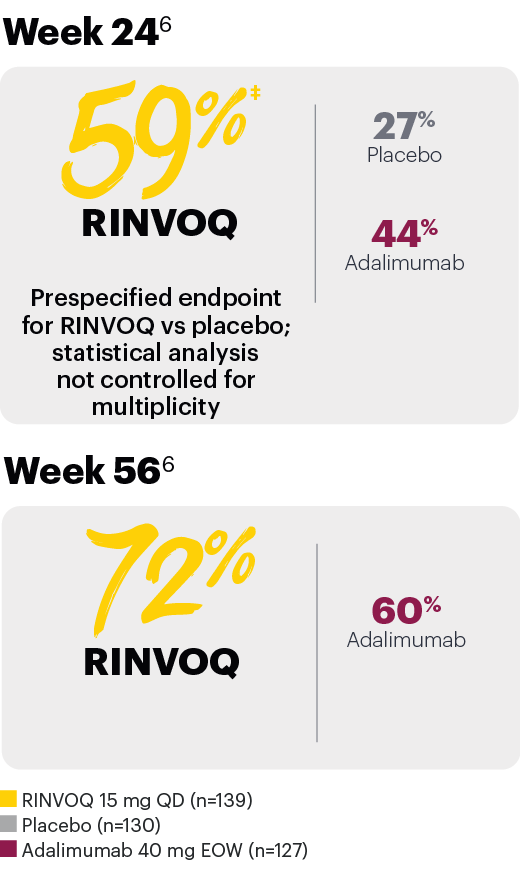
Resolution of dactylitis (LDI=0) (NRI)*
*In subjects with baseline presence of dactylitis.
‡Nominal P≤0.001 vs placebo, not multiplicity-controlled. No clinical inferences can be drawn.
#RINVOQ 15 mg did not meet superiority vs adalimumab for ACR20 response, thus statistical significance vs placebo regarding proportion of patients achieving resolution of dactylitis could not be tested under the hierarchical analysis plan.
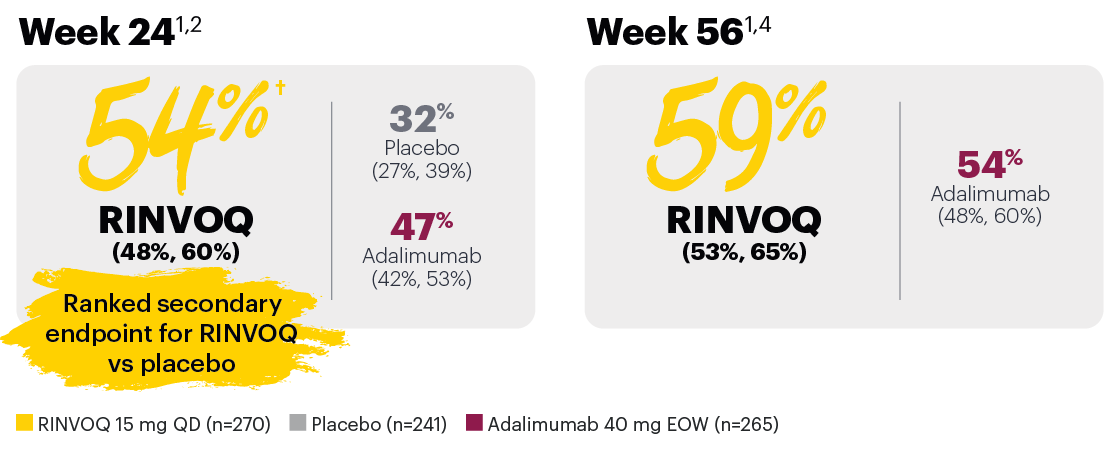
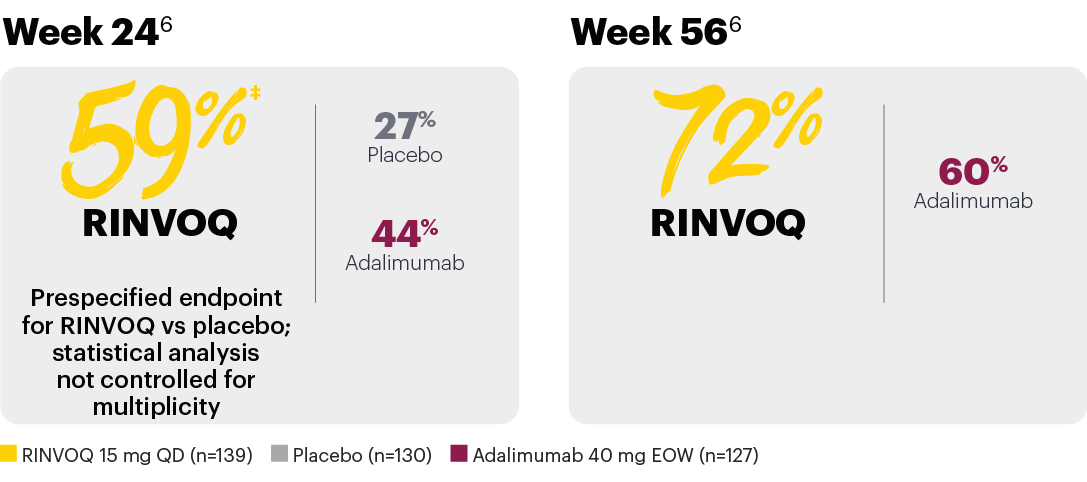
Achievement of BASDAI 50*
In patients with psoriatic spondylitis, RINVOQ showed improvements from baseline in BASDAI scores vs placebo at Week 24.1
*In subjects with baseline presence of psoriatic spondylitis (31% of SELECT-PsA 1 patients).
‡Nominal P≤0.001 vs placebo, not multiplicity-controlled. No clinical inferences can be drawn.
Subjects rescued at Week 16 were imputed as nonresponders in the resolution of enthesitis and dactylitis at Week 24.
DATA LIMITATIONS: Data not labeled as a ranked primary or secondary endpoint were prespecified, however they were not ranked or controlled for multiplicity; therefore, treatment differences could represent chance findings. No conclusions regarding these comparisons can be made.
The BASDAI is composed of 6 items investigating 5 domains (fatigue, spinal pain, joint pain/swelling, areas of localized tenderness, and morning stiffness).7
95% confidence intervals are included in parentheses after each percentage, where available.
Missing data were handled using NRI.
BSA: body surface area; csDMARD: conventional synthetic disease-modifying antirheumatic drug; EOW: every other week; LDI: Leeds Dactylitis Index; LEI: Leeds Enthesitis Index; NRI: nonresponder imputation; PASI 75: Psoriasis Area and Severity Index 75% improvement; QD: once daily.
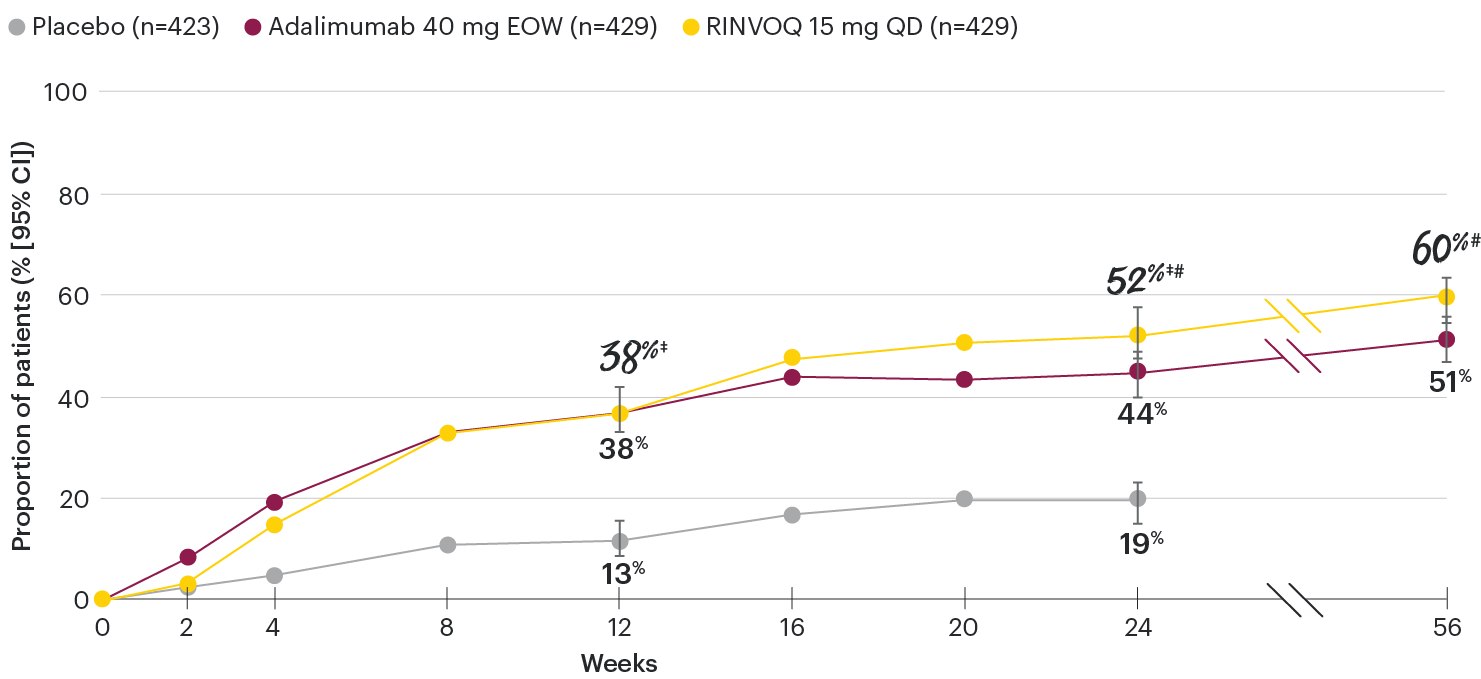
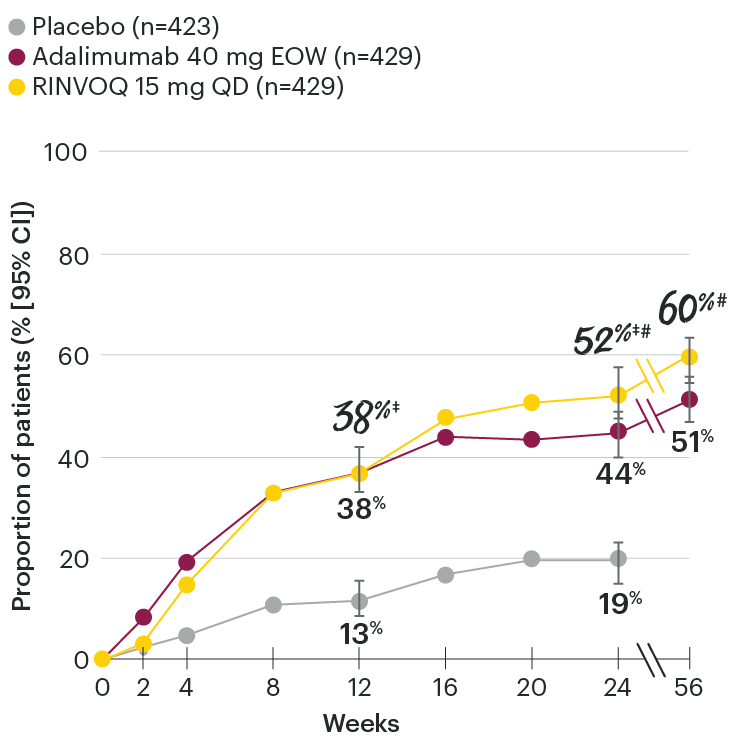
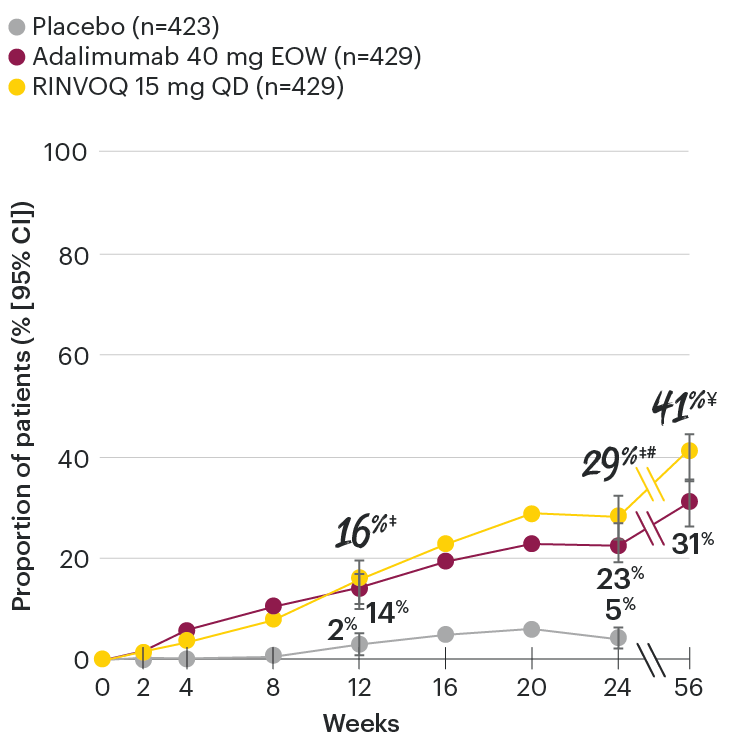
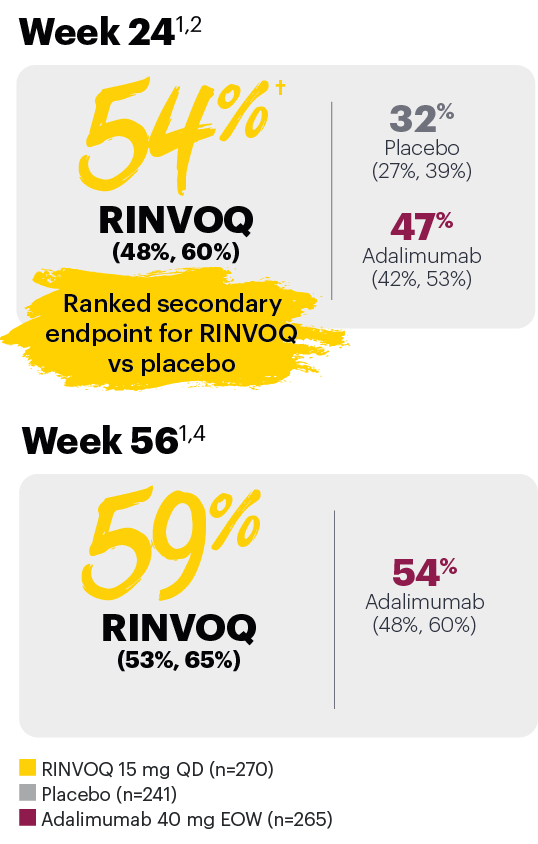
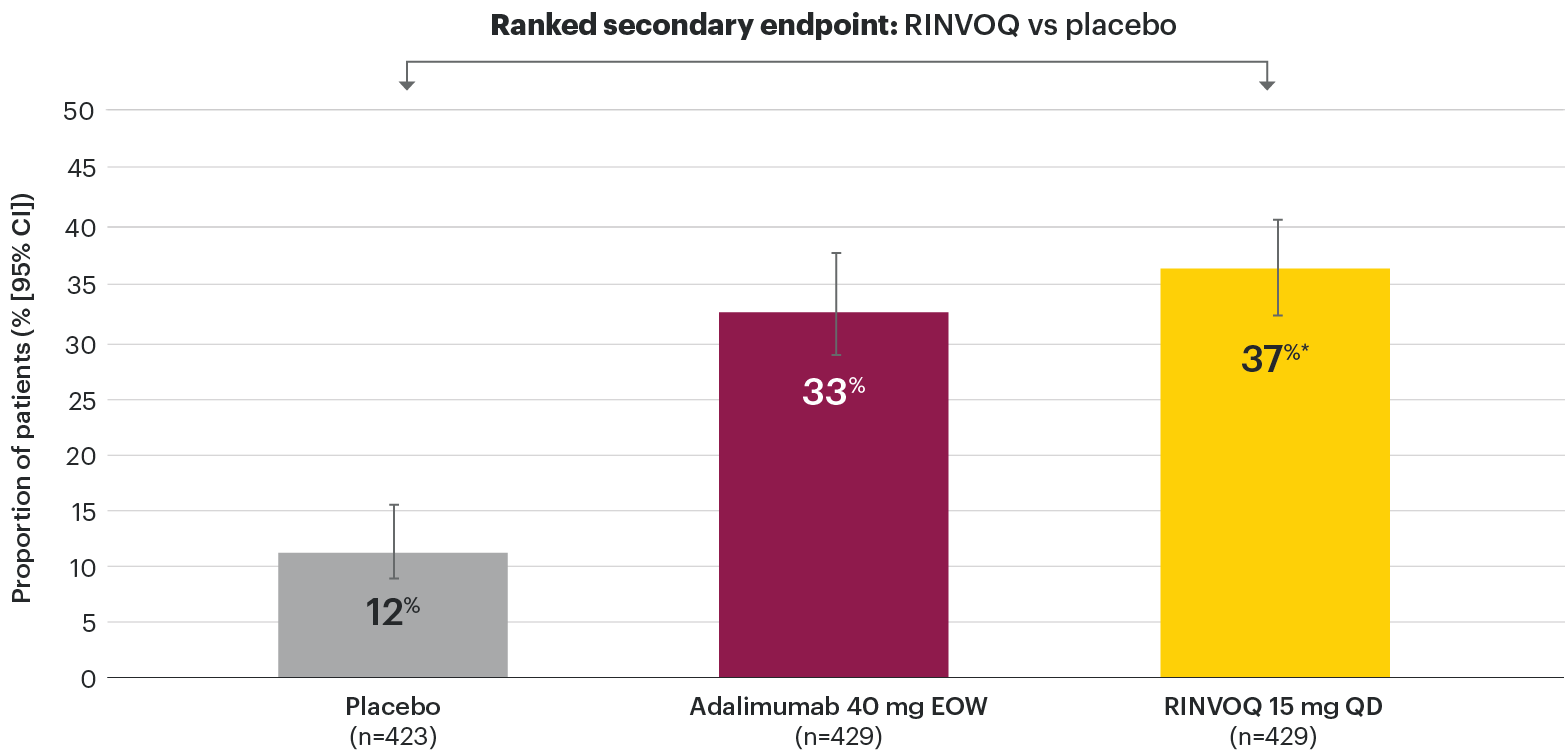
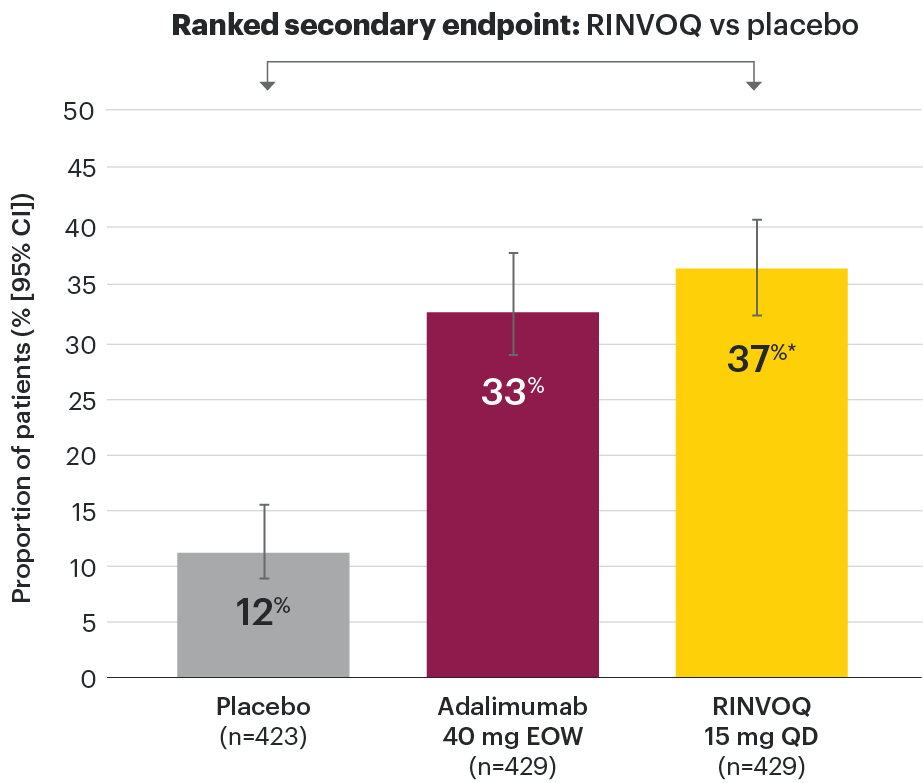
In patients with active PsA and an inadequate response to non-biologic DMARDs
Significantly more patients treated with RINVOQ achieved minimal disease activity vs placebo at Week 24
SELECT-PsA 1: Minimal disease activity† at Week 24 (NRI)1,2
- Tender joint count ≤1
- Swollen joint count ≤1
- PASI ≤1 or BSA-Ps ≤3%
- Patient pain NRS ≤1.5
- Patient Global Disease Activity NRS ≤2
- HAQ-DI ≤0.5
- Tender entheseal points (LEI) ≤1
Subjects rescued at Week 16 were imputed as nonresponders in the MDA analysis.
95% confidence intervals are displayed as error bars in the chart.
Missing data were handled using NRI.
BSA-Ps: body surface area with psoriasis; csDMARD: conventional synthetic disease-modifying antirheumatic drug; EOW: every other week; HAQ-DI: Health Assessment Questionnaire Disability Index; LEI: Leeds Enthesitis Index; NRI: nonresponder imputation; NRS: numeric rating scale; PASI: Psoriasis Area and Severity Index; QD: once daily.
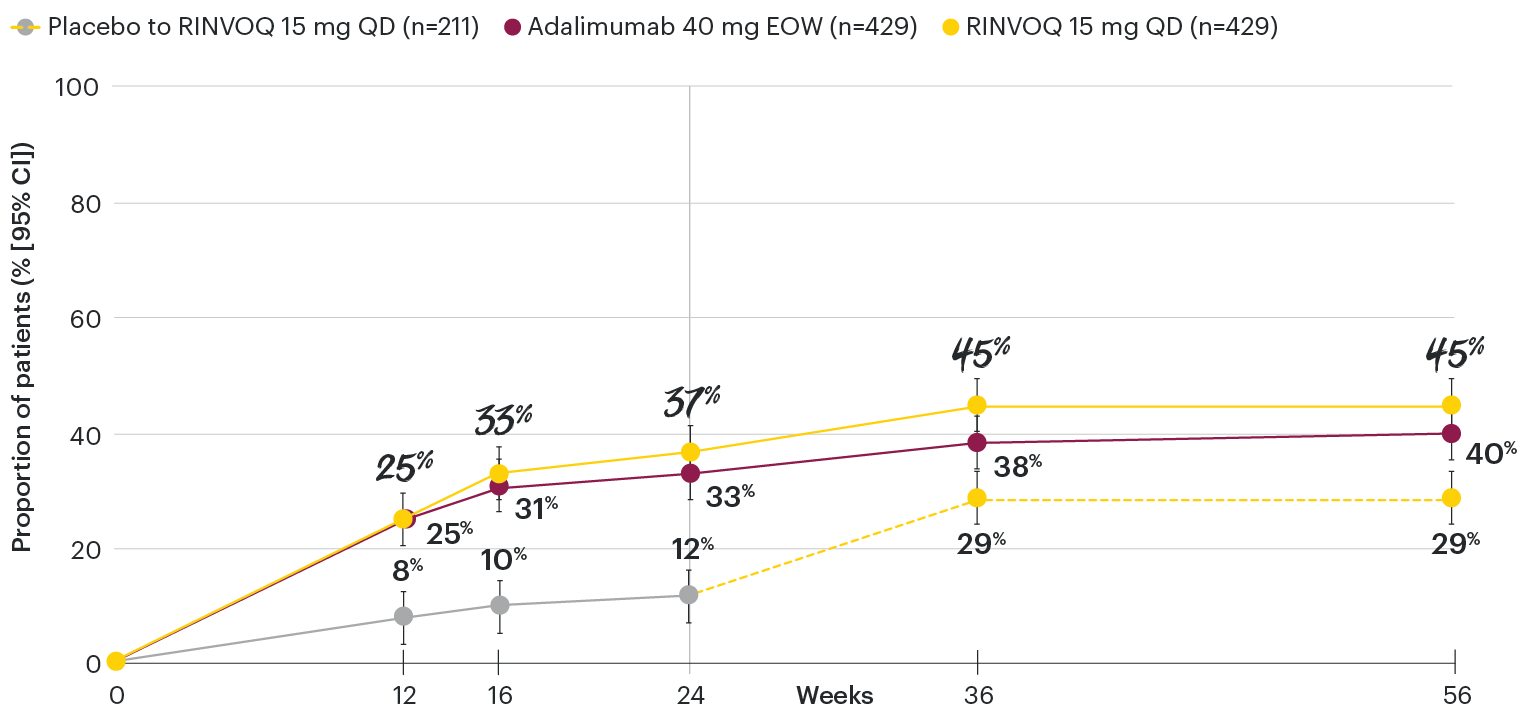
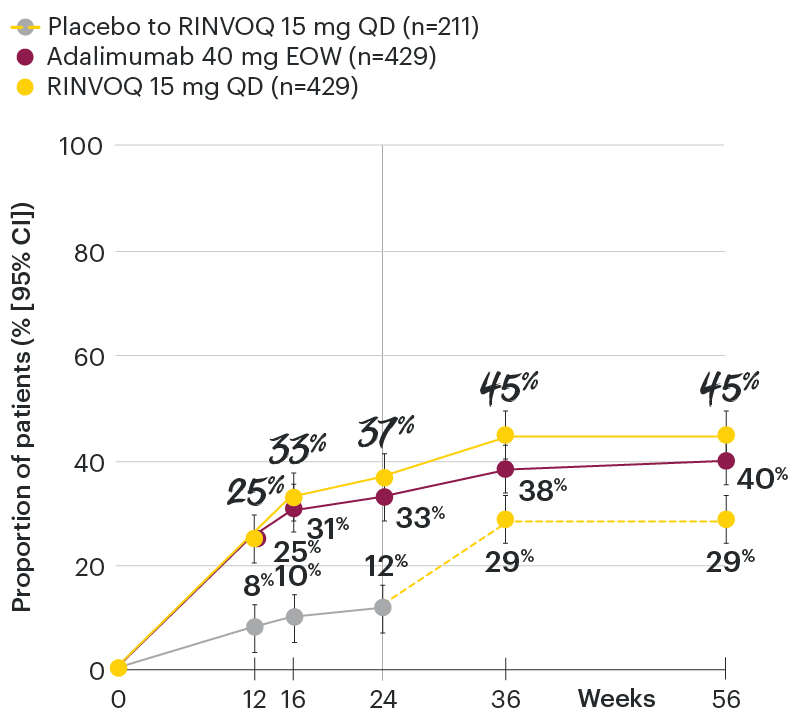
In patients with active PsA and an inadequate response to non-biologic DMARDs
Minimal disease activity over time
SELECT-PsA 1: Minimal disease activity† through Week 56 (NRI)4
DATA LIMITATIONS: Data not labeled as a primary or ranked secondary endpoint were prespecified, however they were not ranked or controlled for multiplicity; therefore, treatment differences could represent chance findings. No conclusions regarding these comparisons can be made.
- Tender joint count ≤1
- Swollen joint count ≤1
- PASI ≤1 or BSA-Ps ≤3%
- Patient pain NRS ≤1.5
- Patient Global Disease Activity NRS ≤2
- HAQ-DI ≤0.5
- Tender entheseal points (LEI) ≤1
At Week 24, all patients randomized to placebo were switched to RINVOQ 15 mg or upadacitinib 30 mg QD in a blinded manner. Upadacitinib 30 mg QD results not provided since 30 mg is not an approved dose in PsA. All data shown are from the Week 56 data cut of SELECT-PsA 1 and may include differences when compared with the primary Week 24 analysis. Subjects rescued at Week 16 were imputed as nonresponders in the minimal disease activity analysis.
95% confidence intervals are displayed as error bars in the chart.
Missing data were handled using NRI.
csDMARD: conventional synthetic disease-modifying antirheumatic drug; EOW: every other week; NRI: nonresponder imputation; QD: once daily.
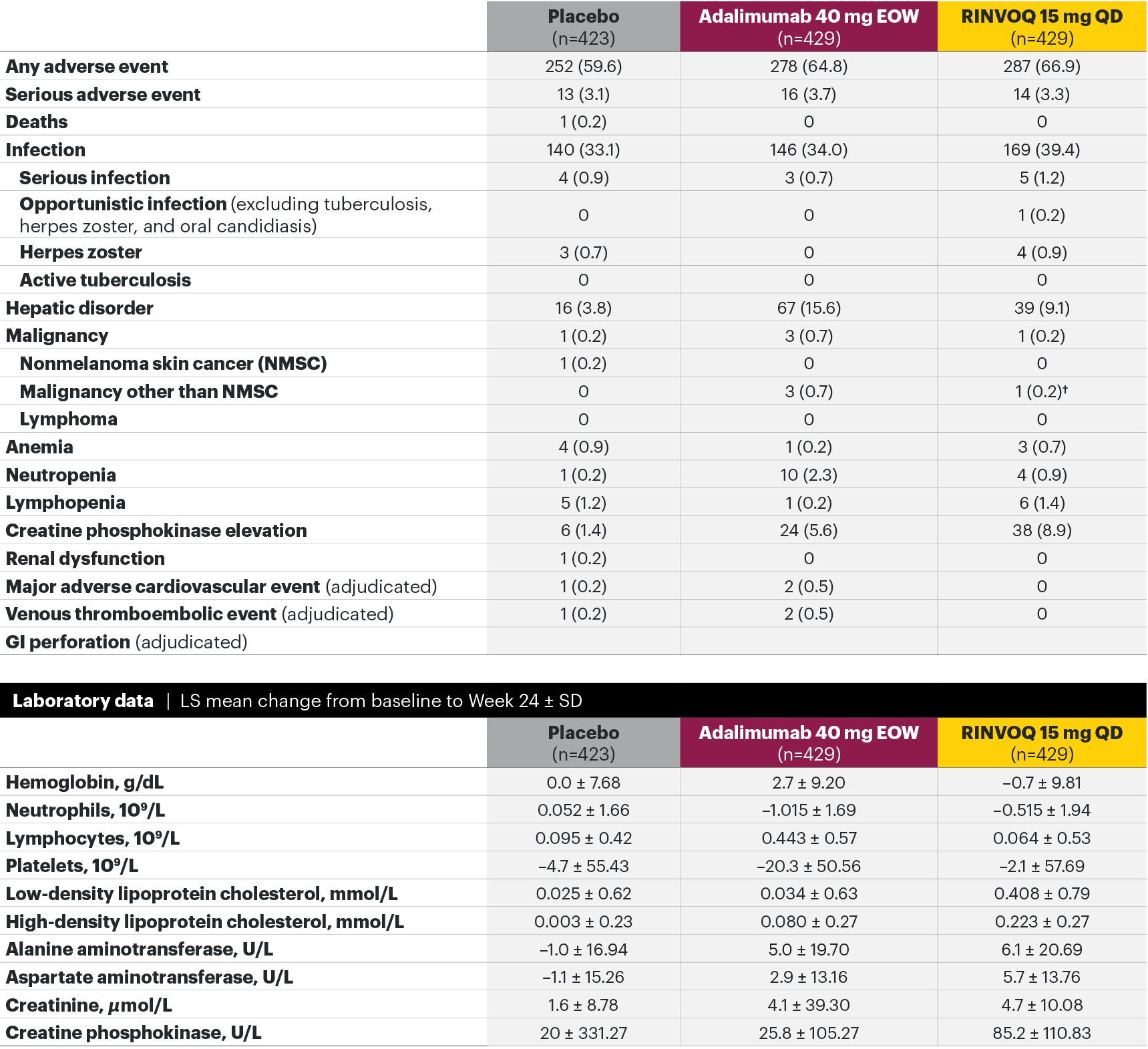
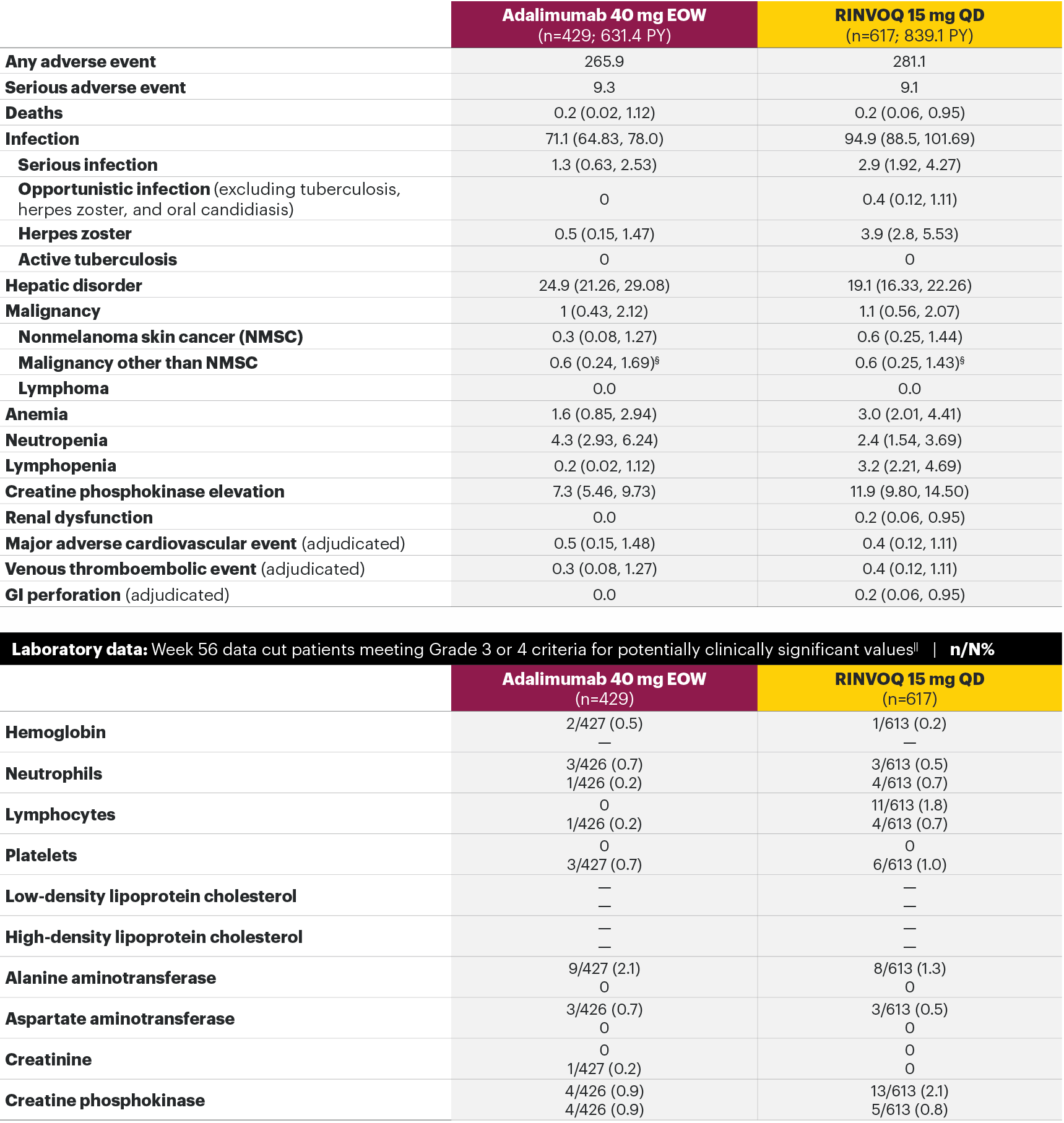
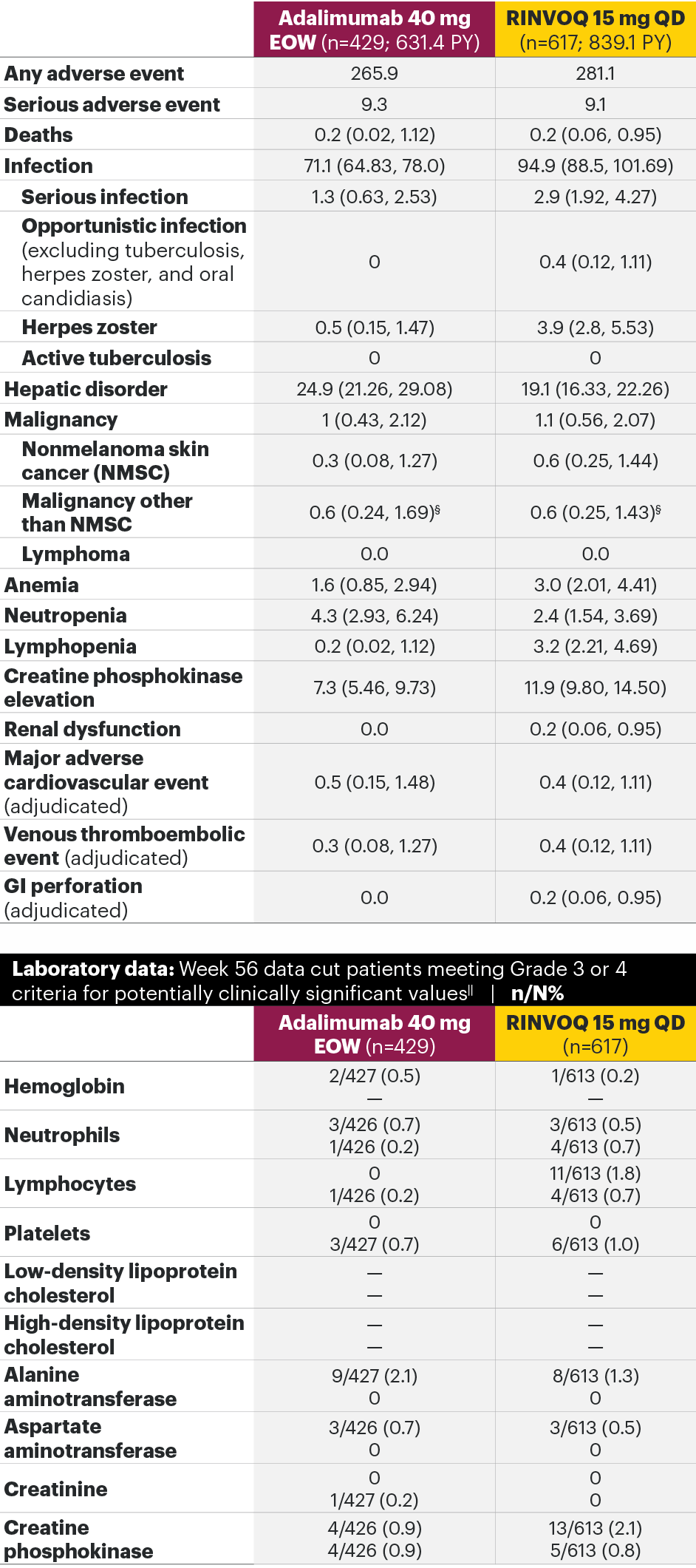
In patients with active PsA and an inadequate response to non-biologic DMARDs
The safety profile of RINVOQ in PsA was consistent with previously reported results in RA1,2,4*
SELECT-PsA 1: Adverse events through Week 24 and the Week 56 data cut2,4**
Most common adverse events: In SELECT-PsA 1, the most common AEs at Week 24 were upper respiratory tract infection, nasopharyngitis, blood CPK increase, ALT increase, and AST increase.2¶ At the Week 56 data cut, the most commonly reported AEs were upper respiratory tract infection and blood CPK elevations.4
For the safety analysis at the Week 56 data cut, the RINVOQ 15-mg group included patients who were originally randomized to placebo and switched to RINVOQ 15 mg at Week 24.
Adverse events leading to discontinuation of study drug occurred in 3.1% of patients in the placebo group, 5.1% in the adalimumab 40-mg-EOW group, and 3.0% in the RINVOQ 15-mg-QD group through Week 242 and in 5.4% in the adalimumab 40-mg-EOW group and 4.9% in the RINVOQ 15-mg-QD group at the Week 56 data cut.4
*A higher rate of serious infections (2.6 events per 100 patient-years and 1.3 events per 100 patient-years, respectively) and hepatic transaminase elevations (ALT elevations Grade 3 and higher rates 1.4% and 0.4%, respectively) was observed in patients treated with RINVOQ in combination with MTX therapy compared to patients treated with monotherapy.1
†Lung cancer metastasis.
‡At the Week 56 data cut, NMSC, malignancy other than NMSC, MACE, VTEs, and death were reported as exposure-adjusted incidence rates (EAIR). All other adverse events at the Week 56 data cut were reported as exposure-adjusted event rates (EAER).
§There were 11 malignancies reported in the RINVOQ 15-mg group (4 basal cell carcinomas, 2 squamous cell carcinoma of skin, and 1 event each of endometrial adenocarcinoma, lung adenocarcinoma, lung cancer metastatic, malignant melanoma, and neuroendocrine carcinoma) and 6 malignancies reported with adalimumab (2 basal cell carcinomas, and 1 event each of colon cancer metastatic, ovarian cancer, pancreatic carcinoma metastatic, and uterine cancer).
IIHemoglobin: Grade 3 (<80 g/dL); neutrophils: Grade 3 (0.5–<1.0 x 109/L), Grade 4 (<0.5 x 109/L); lymphocytes: Grade 3 (0.2–<0.5x 109/L), Grade 4 (<0.2 x 109/L); platelets: Grade 3 (25–<50 x 109/L), Grade 4 (<25 x 109/L); alanine aminotransferase (U/L) and aspartate aminotransferase (U/L): Grade 3 (>5.0–20.0xULN), Grade 4 (>20.0xULN); creatinine (µmol/L): Grade 3 (>3.0–6.0xULN or >3.0xbaseline), Grade 4 (>6.0xULN); creatine phosphokinase (U/L): Grade 3 (>5.0xULN–10.0xULN), Grade 4 (>10.0xULN).
¶Treatment-emergent AEs reported in ≥5% of patients in any treatment arm through Week 24.
**Some patients had longer than 56 weeks of exposure at this data cut.
AE: adverse event; ALT: alanine aminotransferase; EOW: every other week; MACE: major adverse cardiovascular event; NMSC: nonmelanoma skin cancer; QD: once daily; ULN: upper limit of normal; VTE: venous thromboembolic event.
RINVOQ Important Safety Information1
Contraindications
RINVOQ is contraindicated in patients hypersensitive to the active substance or to any of the excipients, in patients with active tuberculosis (TB) or active serious infections, in patients with severe hepatic impairment, and during pregnancy.
Special warnings and precautions for use
Immunosuppressive medicinal products
Use in combination with other potent immunosuppressants is not recommended.
Serious infections
Serious and sometimes fatal infections have been reported in patients receiving upadacitinib. The most frequent serious infections reported included pneumonia and cellulitis. Cases of bacterial meningitis have been reported. Among opportunistic infections, TB, multidermatomal herpes zoster, oral/esophageal candidiasis, and cryptococcosis have been reported with upadacitinib. As there is a higher incidence of infections in patients ≥65 years of age, caution should be used when treating this population.
Viral reactivation
Viral reactivation, including cases of herpes zoster, was reported in clinical studies. The risk of herpes zoster appears to be higher in Japanese patients treated with upadacitinib.
Vaccinations
The use of live, attenuated vaccines during or immediately prior to therapy is not recommended. It is recommended that patients be brought up to date with all immunizations, including prophylactic zoster vaccinations, prior to initiating upadacitinib, in agreement with current immunization guidelines.
Malignancy
The risk of malignancies, including lymphoma is increased in patients with rheumatoid arthritis (RA). Malignancies, including nonmelanoma skin cancer (NMSC), have been reported in patients treated with upadacitinib. Consider the risks and benefits of upadacitinib treatment prior to initiating therapy in patients with a known malignancy other than a successfully treated NMSC or when considering continuing upadacitinib therapy in patients who develop a malignancy.
Hematological abnormalities
Treatment should not be initiated, or should be temporarily interrupted, in patients with hematological abnormalities observed during routine patient management.
Cardiovascular risk
RA patients have an increased risk for cardiovascular disorders. Patients treated with upadacitinib should have risk factors (e.g., hypertension, hyperlipidemia) managed as part of usual standard of care.
Lipids
Upadacitinib treatment was associated with dose-dependent increases in lipid parameters, including total cholesterol, low-density lipoprotein cholesterol, and high-density lipoprotein cholesterol.
Hepatic transaminase elevations
Treatment with upadacitinib was associated with an increased incidence of liver enzyme elevation compared to placebo.
Venous thromboembolisms
Events of deep vein thrombosis (DVT) and pulmonary embolism (PE) have been reported in patients receiving JAK inhibitors, including upadacitinib. Upadacitinib should be used with caution in patients at high risk for DVT/PE.
Adverse reactions
The most commonly reported adverse reactions in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis clinical trials (≥2% of patients in at least one of the indications) with upadacitinib 15 mg were upper respiratory tract infections, blood creatine phosphokinase (CPK) increased, alanine transaminase (ALT) increased, bronchitis, nausea, cough, aspartate transaminase (AST) increased, and hypercholesterolemia. The most common serious adverse reactions were serious infections.
The safety profile of upadacitinib with long term treatment was generally similar to the safety profile during the placebo-controlled period across indications.
Overall, the safety profile observed in patients with psoriatic arthritis or active ankylosing spondylitis treated with upadacitinib 15 mg was consistent with the safety profile observed in patients with RA.
This is not a complete summary of all safety information.
Please see the RINVOQ Summary of Product Characteristics for complete prescribing information.
[Insert local HUMIRA Important Safety Information]
Please see the HUMIRA Summary of Product Characteristics for complete prescribing information.
NOTE TO AFFILIATES: Consult local MRL regarding adding a link to the adalimumab SmPC in the main navigation area.
- RINVOQ [Summary of Product Characteristics]. AbbVie Deutschland GmbH & Co. KG.
- McInnes IB, Anderson JK, Magrey M, et al. Trial of upadacitinib and adalimumab for psoriatic arthritis. N Engl J Med. 2021;384(13):1227-1239. doi:10.1056/NEJMoa2022516
- Data on File, AbbVie Inc. ABVRRTI71394 [Week 24 data].
- McInnes IB, Kato K, Magrey M, et al. Upadacitinib in patients with psoriatic arthritis and an inadequate response to non-biological therapy: 56-week data from the phase 3 SELECT-PsA 1 study. RMD Open. 2021;7(3):e001838. doi:10.1136/rmdopen-2021-001838
- Data on File, AbbVie Inc. ABVRRTI71396.
- European Medicines Agency (EMA). RINVOQ assessment report - variation. https://www.ema.europa.eu/en/documents/variation-report/rinvoq-h-c-004760-ii-0004-epar-assessment-report-variation_en.pdf. Updated March 2021. Accessed May 10, 2021.
- Zochling J. Measures of symptoms and disease status in ankylosing spondylitis: Ankylosing Spondylitis Disease Activity Score (ASDAS), Ankylosing Spondylitis Quality of Life Scale (ASQoL), Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), Bath Ankylosing Spondylitis Functional Index (BASFI), Bath Ankylosing Spondylitis Global Score (BAS-G), Bath Ankylosing Spondylitis Metrology Index (BASMI), Dougados Functional Index (DFI), and Health Assessment Questionnaire for the Spondylarthropathies (HAQ-S). Arthritis Care Res (Hoboken). 2011;63(suppl 11):S47-S58.
- Coates LC, Fransen J, Helliwell PS. Defining minimal disease activity in psoriatic arthritis: a proposed objective target for treatment. Ann Rheum Dis. 2010;69(1):48-53. doi:10.1136/ard.2008.102053