Expert perspectives
RINVOQ Important Safety Information1
Contraindications
RINVOQ is contraindicated in patients hypersensitive to the active substance or to any of the excipients, in patients with active tuberculosis (TB) or active serious infections, in patients with severe hepatic impairment, and during pregnancy.
Special warnings and precautions for use
Immunosuppressive medicinal products
Use in combination with other potent immunosuppressants is not recommended.
Serious infections
Serious and sometimes fatal infections have been reported in patients receiving upadacitinib. The most frequent serious infections reported included pneumonia and cellulitis. Cases of bacterial meningitis have been reported. Among opportunistic infections, TB, multidermatomal herpes zoster, oral/esophageal candidiasis, and cryptococcosis have been reported with upadacitinib. As there is a higher incidence of infections in patients ≥65 years of age, caution should be used when treating this population.
Viral reactivation
Viral reactivation, including cases of herpes zoster, was reported in clinical studies. The risk of herpes zoster appears to be higher in Japanese patients treated with upadacitinib.
Vaccinations
The use of live, attenuated vaccines during or immediately prior to therapy is not recommended. It is recommended that patients be brought up to date with all immunizations, including prophylactic zoster vaccinations, prior to initiating upadacitinib, in agreement with current immunization guidelines.
Malignancy
The risk of malignancies, including lymphoma is increased in patients with rheumatoid arthritis (RA). Malignancies, including nonmelanoma skin cancer (NMSC), have been reported in patients treated with upadacitinib. Consider the risks and benefits of upadacitinib treatment prior to initiating therapy in patients with a known malignancy other than a successfully treated NMSC or when considering continuing upadacitinib therapy in patients who develop a malignancy.
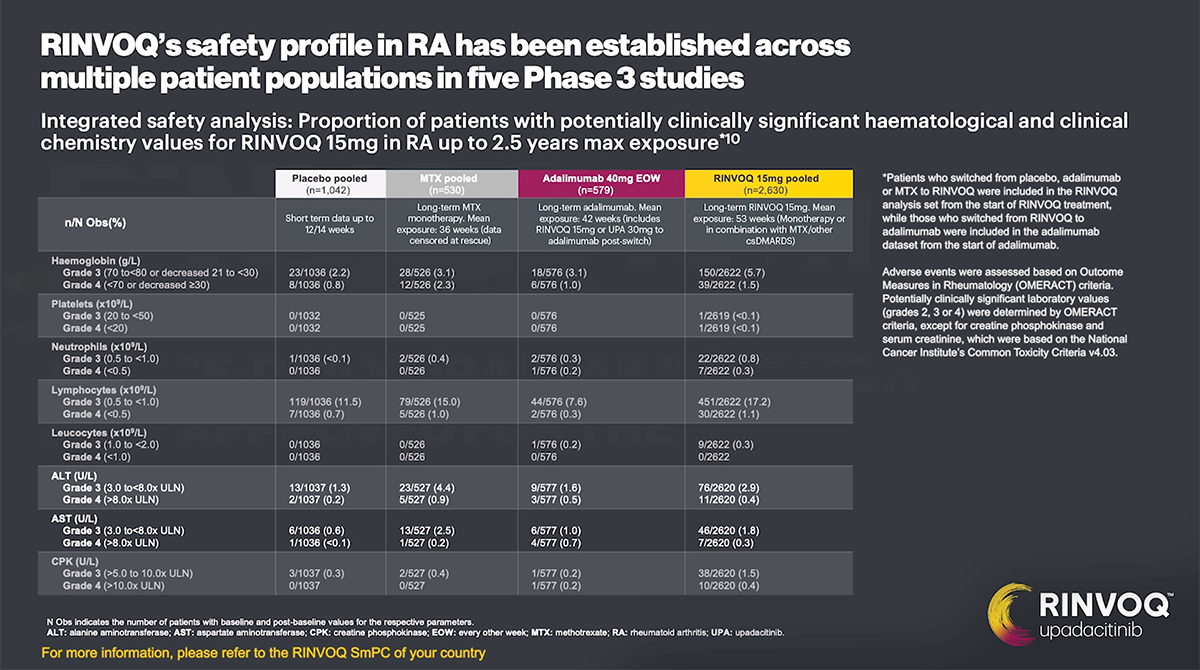
Hematological abnormalities
Treatment should not be initiated, or should be temporarily interrupted, in patients with hematological abnormalities observed during routine patient management.
Cardiovascular risk
RA patients have an increased risk for cardiovascular disorders. Patients treated with upadacitinib should have risk factors (e.g., hypertension, hyperlipidemia) managed as part of usual standard of care.
Lipids
Upadacitinib treatment was associated with dose-dependent increases in lipid parameters, including total cholesterol, low-density lipoprotein cholesterol, and high-density lipoprotein cholesterol.
Hepatic transaminase elevations
Treatment with upadacitinib was associated with an increased incidence of liver enzyme elevation compared to placebo.
Venous thromboembolisms
Events of deep vein thrombosis (DVT) and pulmonary embolism (PE) have been reported in patients receiving JAK inhibitors, including upadacitinib. Upadacitinib should be used with caution in patients at high risk for DVT/PE.
Adverse reactions
The most commonly reported adverse reactions in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis clinical trials (≥2% of patients in at least one of the indications) with upadacitinib 15 mg were upper respiratory tract infections, blood creatine phosphokinase (CPK) increased, alanine transaminase (ALT) increased, bronchitis, nausea, cough, aspartate transaminase (AST) increased, and hypercholesterolemia. The most common serious adverse reactions were serious infections.
The safety profile of upadacitinib with long term treatment was generally similar to the safety profile during the placebo-controlled period across indications.
Overall, the safety profile observed in patients with psoriatic arthritis or active ankylosing spondylitis treated with upadacitinib 15 mg was consistent with the safety profile observed in patients with RA.
This is not a complete summary of all safety information.
Please see the RINVOQ Summary of Product Characteristics for complete prescribing information.
[Insert local HUMIRA Important Safety Information]
Please see the HUMIRA Summary of Product Characteristics for complete prescribing information.
NOTE TO AFFILIATES: Consult local MRL regarding adding a link to the adalimumab SmPC in the main navigation area.
- RINVOQ [Summary of Product Characteristics]. AbbVie Deutschland GmbH & Co. KG.
- McInnes IB, Anderson JK, Magrey M, et al. Trial of upadacitinib and adalimumab for psoriatic arthritis. N Engl J Med. 2021;384(13):1227-1239. doi:10.1056/NEJMoa2022516