Note to Affiliate: This material has been developed using the DRAFT EU SmPC [XXXX VX] as reference. This material must be reviewed against final approved EU labeling once available and revised prior to final review/approve for distribution.
Note to Affiliate: “Now Approved” is a regulated term to be used for up to one year per EFPIA code of practice. Please evaluate use of the term given dates of availability in your country.
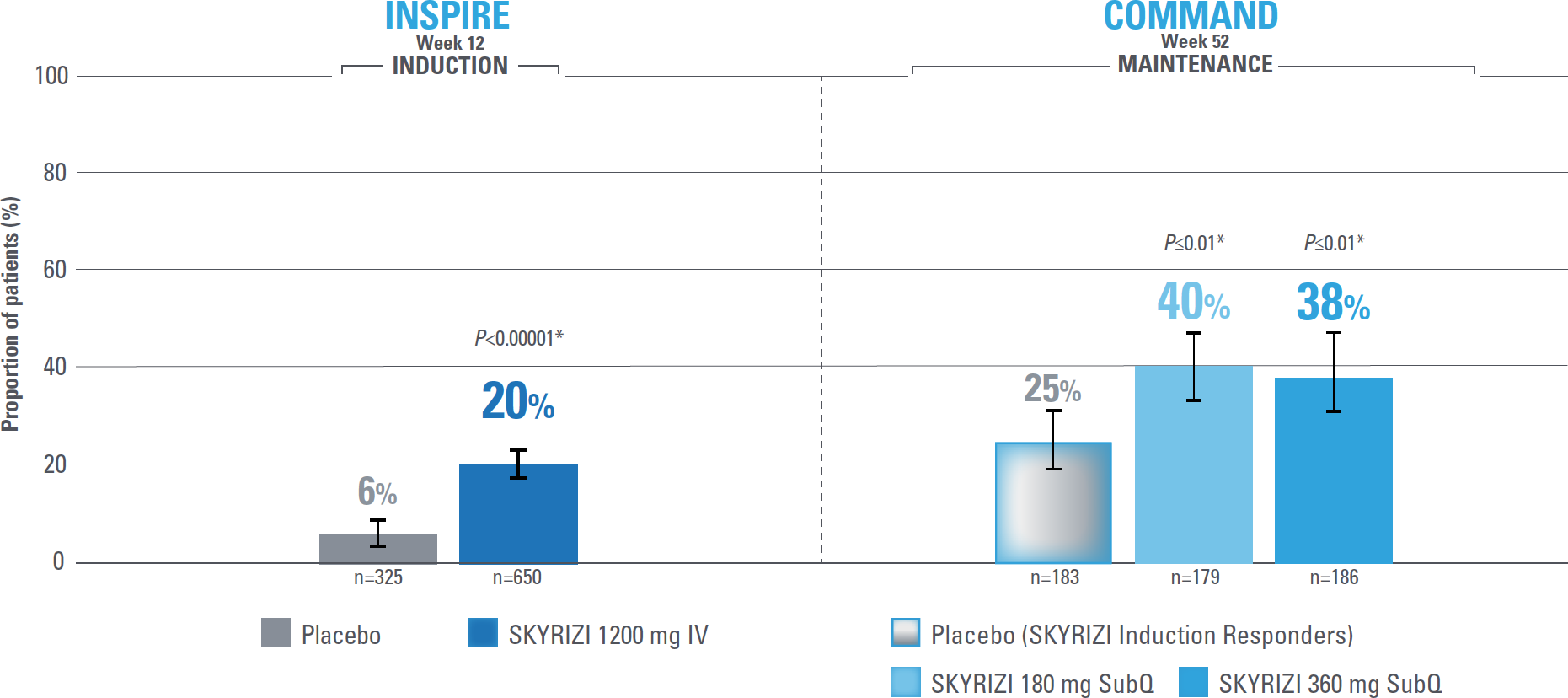
Control is defined by the primary endpoint of clinical remission per modified Mayo score (mMS; INSPIRE: 20% [1200 mg IV]; COMMAND: 40% [180 mg SubQ], 38% [360 mg SubQ]) based on stool frequency subscore (SFS), rectal bleeding subscore (RBS), and endoscopic subscore (ES) at Weeks 12 and 52.1
SKYRIZI CLINICAL TRIALS INCLUDED PRIMARY ENDPOINT OF CLINICAL REMISSION PER mMS AT WEEKS 12 AND 521,2
Mucosal healing at Weeks 12 and 521*
Endoscopic remission at Weeks 12 and 521*
Histologic-endoscopic mucosal healing at Weeks 12 and 521*
Clinical remission at Weeks 12 and 521*
Significant improvement in patient-reported outcomes at Week 121*
Safety through 12 and 52 weeks2
Safety across therapeutic areas1,3
*Statistically significant under multiplicity-control for SKYRIZI vs placebo comparison.1,2
Primary endpoint definition
Clinical remission per mMS: SFS ≤1 and not greater than baseline, RBS=0, and ES ≤1 without evidence of friability.1
Endpoint definitions
Mucosal healing: ES ≤1 without the evidence of friability.1
Endoscopic remission: Normalization of the endoscopic appearance of the mucosa and defined as an ES of 0.1
Histologic-endoscopic mucosal healing: ES ≤1 without the evidence of friability and Geboes score ≤3.1 (indicating neutrophil infiltration in <5% of crypts, no crypt destruction and no erosions, ulcerations, or granulation tissue).1
Primary endpoint of the SKYRIZI UC Phase 3 clinical trials1*
Clinical remission per mMS1,2
Composite of RBS, SFS, and ES
Error bars represent 95% confidence interval.2
*Statistically significant under multiplicity-control for SKYRIZI vs placebo comparison.1,2
IMPORTANT CONTEXT ABOUT Placebo (SKYRIZI induction responders): The placebo group in the maintenance study COMMAND consisted of subjects who achieved clinical response to risankizumab induction therapy and were randomized to receive placebo in the maintenance study (COMMAND). Therefore, the placebo arm is referred to as “Placebo (SKYRIZI Induction Responders)” in the maintenance study sections within this site and referred to as “SKYRIZI IV/Placebo SC” in the SmPC.1
Primary endpoint definition
Clinical remission per mMS: SFS ≤1 and not greater than baseline, RBS=0, and ES ≤1 without evidence of friability.1
Mucosal healing1,2
Week 12 Induction study
INSPIRE—placebo (n=325): 12% of patients; SKYRIZI 1200 mg IV (n=650): 37% of patients. Statistically significant under multiplicity-control for SKYRIZI vs placebo comparison (P<0.00001).1,2
Week 52 Maintenance study
COMMAND—placebo (SKYRIZI Induction Responders, n=183): 32% of patients; SKYRIZI 180 mg SubQ (n=179): 51% of patients; SKYRIZI 360 mg SubQ (n=186): 48% of patients. Statistically significant under multiplicity-control for SKYRIZI vs placebo comparison (P≤0.01).1,2
Note to Affiliate: “Endoscopic subscore ≤1 without the evidence of friability” is defined as “mucosal healing” in the SmPC and “endoscopic improvement” in Louis et al.
Endoscopic remission1,2
Week 12 Induction study
INSPIRE—placebo (n=325): 3% of patients; SKYRIZI 1200 mg IV (n=650): 11% of patients. Statistically significant under multiplicity-control for SKYRIZI vs placebo comparison (P<0.00001).1,2
Week 52 Maintenance study
COMMAND—placebo (SKYRIZI Induction Responders, n=183): 15% of patients; SKYRIZI 180 mg SubQ (n=179): 23% of patients; SKYRIZI 360 mg SubQ (n=186): 24% of patients. Statistically significant under multiplicity-control for SKYRIZI vs placebo comparison (P<0.05).1,2
Histologic-endoscopic mucosal healing1,2
Week 12 Induction study
INSPIRE—placebo (n=325): 8% of patients; SKYRIZI 1200 mg IV (n=650): 24% of patients. Statistically significant under multiplicity-control for SKYRIZI vs placebo comparison (P<0.00001).1,2
Week 52 Maintenance study
COMMAND—placebo (SKYRIZI Induction Responders, n=183): 23% of patients; SKYRIZI 180 mg SubQ (n=179): 43% of patients; SKYRIZI 360 mg SubQ (n=186): 42% of patients. Statistically significant under multiplicity-control for SKYRIZI vs placebo comparison (P≤0.01).1,2
IMPORTANT CONTEXT ABOUT Placebo (SKYRIZI induction responders): The placebo group in the maintenance study COMMAND consisted of subjects who achieved clinical response to risankizumab induction therapy and were randomized to receive placebo in the maintenance study (COMMAND). Therefore, the placebo arm is referred to as “Placebo (SKYRIZI Induction Responders)” in the maintenance study sections within this site and referred to as “SKYRIZI IV/Placebo SC” in the SmPC.1
Indication1
SKYRIZI is indicated for the treatment of adult patients with moderately to severely active ulcerative colitis who have had an inadequate response to, lost response to, or were intolerant to conventional therapy or a biologic therapy.
Important Safety Information1
Affiliate to insert local ISI.
Risankizumab is contraindicated in patients hypersensitive to the active substance or to any of the excipients, and in patients with clinically important active infections (e.g. active tuberculosis).
Risankizumab may increase the risk of infection. In patients with a chronic infection, a history of recurrent infection, or known risk factors for infection, risankizumab should be used with caution. Treatment with risankizumab should not be initiated in patients with any clinically important active infection until the infection resolves or is adequately treated.
Patients treated with risankizumab should be instructed to seek medical advice if signs or symptoms of clinically important chronic or acute infection occur. If a patient develops such an infection or is not responding to standard therapy for the infection, the patient should be closely monitored and risankizumab should not be administered until the infection resolves.
Prior to initiating treatment with risankizumab, patients should be evaluated for tuberculosis (TB) infection. Patients receiving risankizumab should be monitored for signs and symptoms of active TB. Anti-TB therapy should be considered prior to initiating risankizumab in patients with a past history of latent or active TB in whom an adequate course of treatment cannot be confirmed.
Prior to initiating therapy with risankizumab, completion of all appropriate immunisations should be considered according to current immunisation guidelines. If a patient has received live vaccination (viral or bacterial), it is recommended to wait at least 4 weeks prior to starting treatment with risankizumab. Patients treated with risankizumab should not receive live vaccines during treatment and for at least 21 weeks after treatment.
Serious hypersensitivity reactions, including anaphylaxis, have been reported with use of risankizumab. If a serious hypersensitivity reaction occurs, administration of risankizumab should be discontinued immediately and appropriate therapy initiated.
The most frequently reported adverse reactions were upper respiratory infections (13% in psoriasis, 15.6% in Crohn’s disease and 26.2% in ulcerative colitis).
Commonly (≥ 1/100 to < 1/10) reported adverse reactions included tinea infections, headache, pruritus, rash, eczema, fatigue, and injection site reactions.
This is not a complete summary of all safety information.
See SKYRIZI full Summary of Product Characteristics (SmPC) at www.ema.europa.eu
Globally, prescribing information varies; refer to the individual country product label for complete information.
ES: endoscopic subscore; IV: intravenous; JAK: Janus kinase; mMS: modified Mayo score; RBS: rectal bleeding subscore; SC: subcutaneous; SFS: stool frequency subscore; SmPC: Summary of Product Characteristics; SubQ: subcutaneous.
References: 1. SKYRIZI [Summary of Product Characteristics]. AbbVie Deutschland GmbH & Co. KG; [DRAFT]. 2. Louis E, Schreiber S, Panaccione R, et al. Risankizumab as ulcerative colitis induction and maintenance therapy: INSPIRE and COMMAND studies. A primary analysis of phase 2b/3 randomized clinical trials. DRAFT. 3. Gordon KB, Blauvelt A, Bachelez H, et al. Long-term safety of risankizumab in patients with psoriatic disease: findings from integrated analyses of 17 clinical trials in psoriasis and 4 in psoriatic arthritis. Poster presented at: European Congress of Rheumatology; June 1-4, 2022; Copenhagen, Denmark.