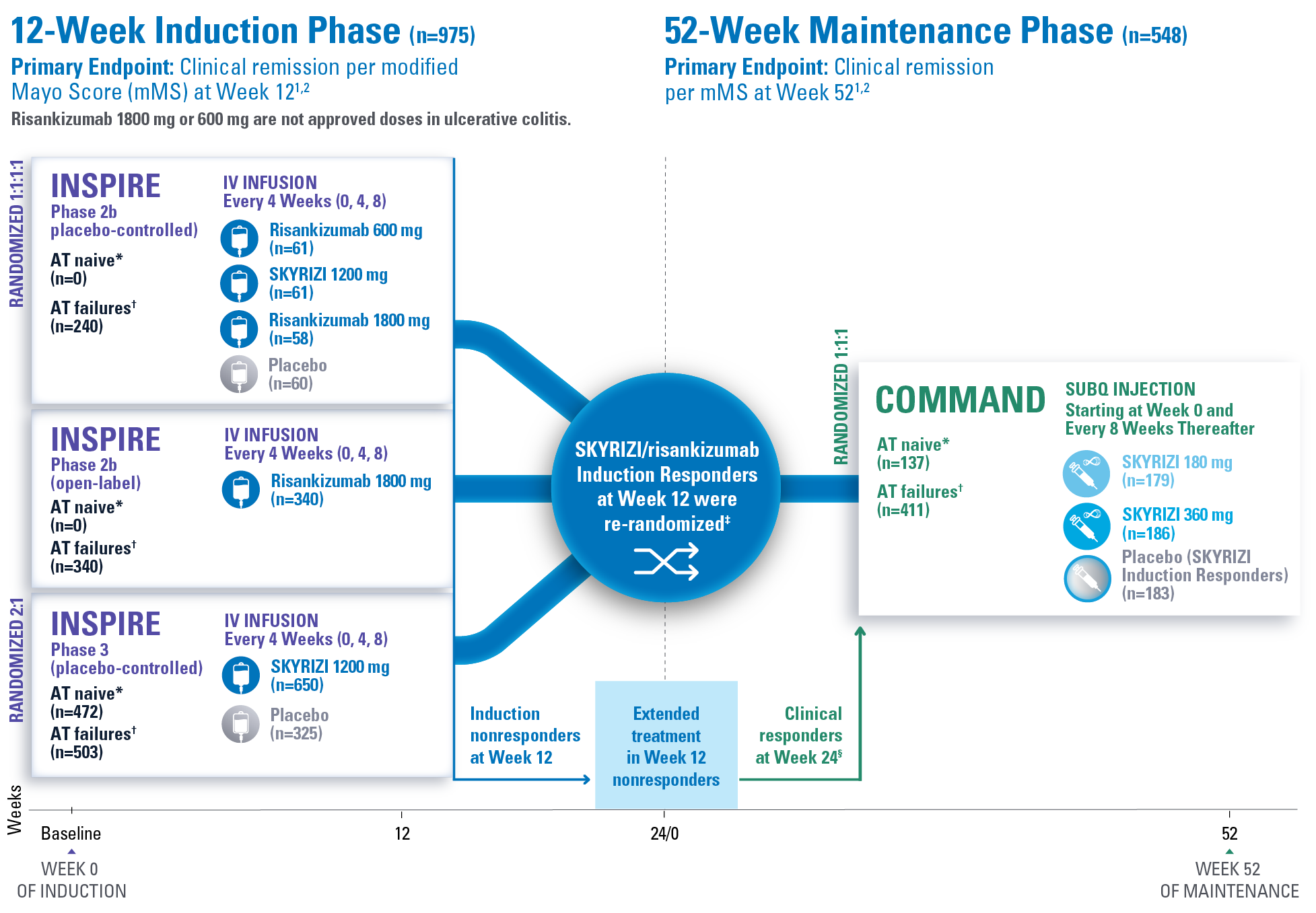
STUDY DESIGN:
INSPIRE and COMMAND1,2
Risankizumab 1800 mg or 600 mg are not approved doses in ulcerative colitis.
Extended treatment in Week 12 nonresponders (n=141):
Patients who did not respond at Week 12 received extended treatment of SKYRIZI (labeled dosing 180 mg or 360 mg) SubQ up to Week 24.1
Primary endpoint definition
Clinical remission per mMS: Stool frequency subscore (SFS) ≤1 and not greater than baseline, rectal bleeding subscore (RBS)=0, and endoscopic subscore (ES) ≤1 without evidence of friability.1
*Advanced therapy naive (AT naive) (subjects without biologic and/or JAK inhibitor failure) are defined as subjects who had not failed (inadequate response or intolerance) one or more biologic therapies, JAK inhibitors, and/or sphingosine 1-phosphate (S1P) receptor modulators.1
†Advanced therapy failures (AT failures) (subjects with biologic and/or JAK inhibitor failure) are defined as subjects who had failed (inadequate response or intolerance) one or more biologic therapies, JAK inhibitors, and/or S1P receptor modulators.1
‡Clinical response per mMS: Decrease from baseline ≥2 points and ≥30% and a decrease in RBS ≥1 or an absolute RBS ≤1.1
§Patients from the Week 24 clinical responders are excluded from the COMMAND primary analysis.2
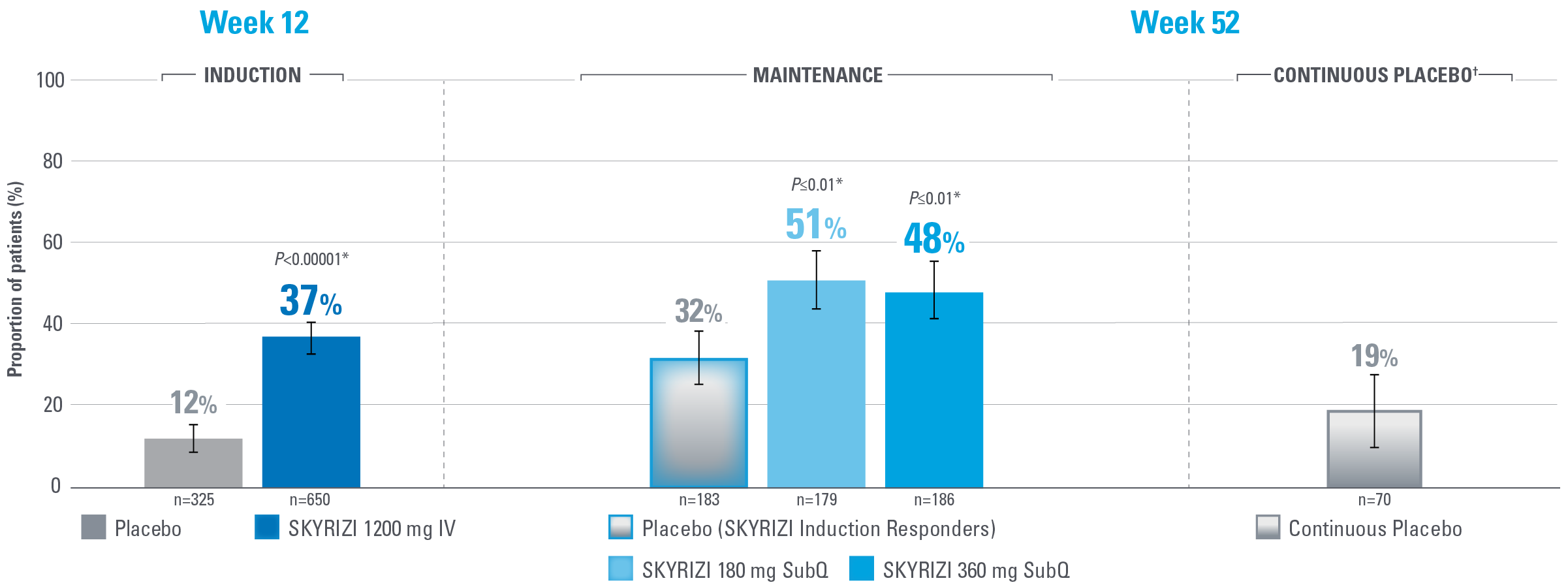
MUCOSAL HEALING ACHIEVED AT WEEKS 12 AND 521,2
Defined as an ES ≤1 without evidence of friability
Note to Affiliate: “Endoscopic subscore ≤1 without the evidence of friability” is defined as “mucosal healing” in the SmPC and “endoscopic improvement” in Louis et al.
Error bars represent 95% confidence interval.2
*Statistically significant under multiplicity-control for SKYRIZI vs placebo comparison.1,2
†Patients on placebo who responded to induction continued on placebo in maintenance and were not included in the primary efficacy analysis. Continuous placebo data not intended for direct comparison.2
IMPORTANT CONTEXT ABOUT Placebo (SKYRIZI Induction Responders) The placebo group in the maintenance study COMMAND consisted of subjects who achieved clinical response to risankizumab induction therapy and were randomized to receive placebo in the maintenance study (COMMAND). Therefore, the placebo arm is referred to as “Placebo (SKYRIZI Induction Responders)” in the maintenance study sections within this site and referred to as “SKYRIZI IV/Placebo SC” in the SmPC.1
Endpoint definition
Mucosal healing: ES ≤1 without evidence of friability.1
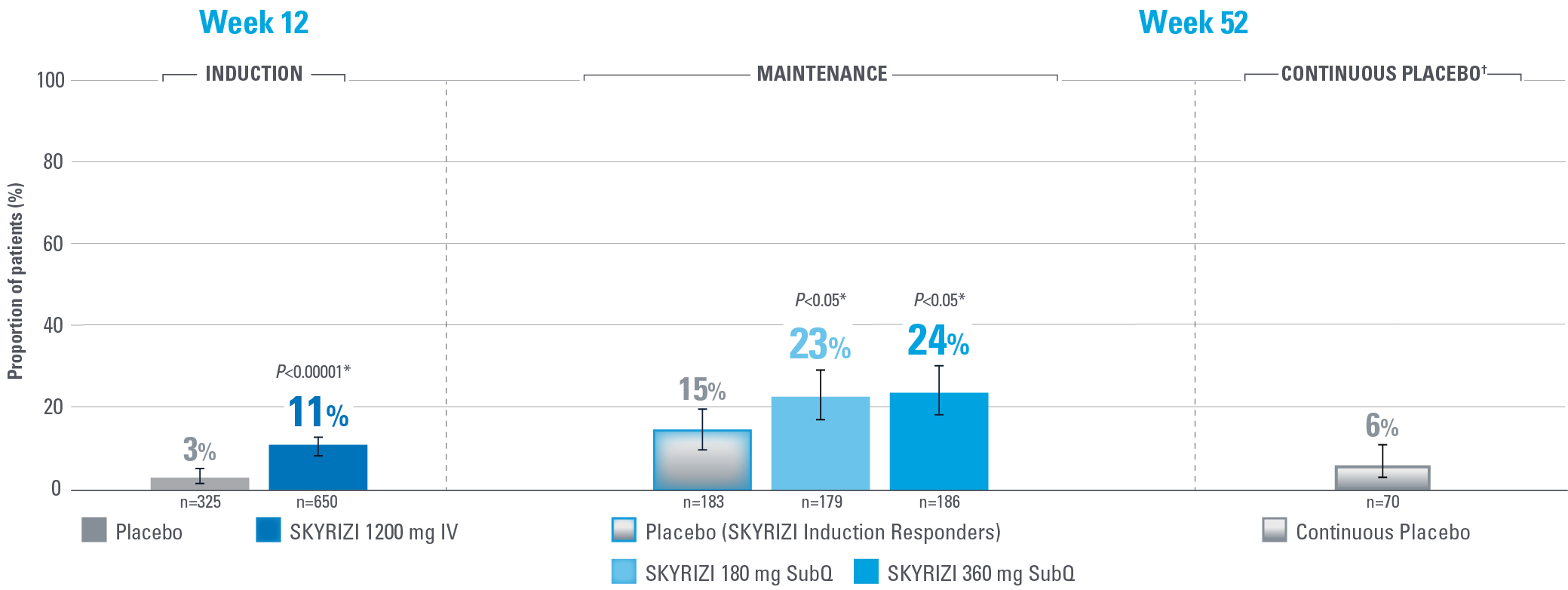
SIGNIFICANT ENDOSCOPIC REMISSION ACHIEVED AT WEEKS 12 AND 521,2
Defined as an ES of 0
Error bars represent 95% confidence interval.2
*Statistically significant under multiplicity-control for SKYRIZI vs placebo comparison.1,2
†Patients on placebo who responded to induction continued on placebo in maintenance and were not included in the primary efficacy analysis. Continuous placebo data not intended for direct comparison.2
IMPORTANT CONTEXT ABOUT Placebo (SKYRIZI Induction Responders) The placebo group in the maintenance study COMMAND consisted of subjects who achieved clinical response to risankizumab induction therapy and were randomized to receive placebo in the maintenance study (COMMAND). Therefore, the placebo arm is referred to as “Placebo (SKYRIZI Induction Responders)” in the maintenance study sections within this site and referred to as “SKYRIZI IV/Placebo SC” in the SmPC.1
Endpoint definition
Endoscopic remission: Normalization of the endoscopic appearance of the mucosa and defined as an ES of 0.1
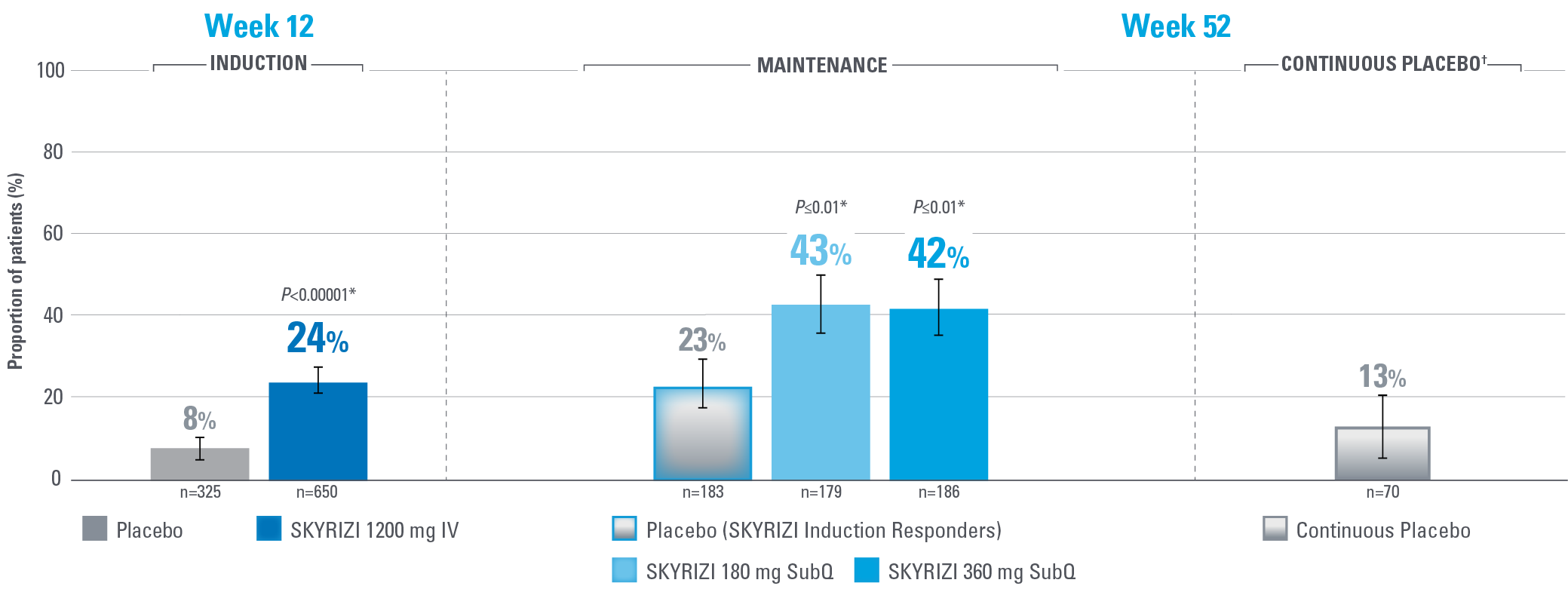
HISTOLOGIC-ENDOSCOPIC MUCOSAL HEALING (HEMH) AT WEEKS 12 AND 521,2
Defined as an ES ≤1 without the evidence of friability and Geboes score ≤3.1
Error bars represent 95% confidence interval.2
*Statistically significant under multiplicity-control for SKYRIZI vs placebo comparison.1,2
†Patients on placebo who responded to induction continued on placebo in maintenance and were not included in the primary efficacy analysis. Continuous placebo data not intended for direct comparison.2
IMPORTANT CONTEXT ABOUT Placebo (SKYRIZI Induction Responders) The placebo group in the maintenance study COMMAND consisted of subjects who achieved clinical response to risankizumab induction therapy and were randomized to receive placebo in the maintenance study (COMMAND). Therefore, the placebo arm is referred to as “Placebo (SKYRIZI Induction Responders)” in the maintenance study sections within this site and referred to as “SKYRIZI IV/Placebo SC” in the SmPC.1
Endpoint definition
HEMH: ES ≤1 without the evidence of friability and Geboes score ≤3.1 (indicating neutrophil infiltration in <5% of crypts, no crypt destruction and no erosions, ulcerations, or granulation tissue).1
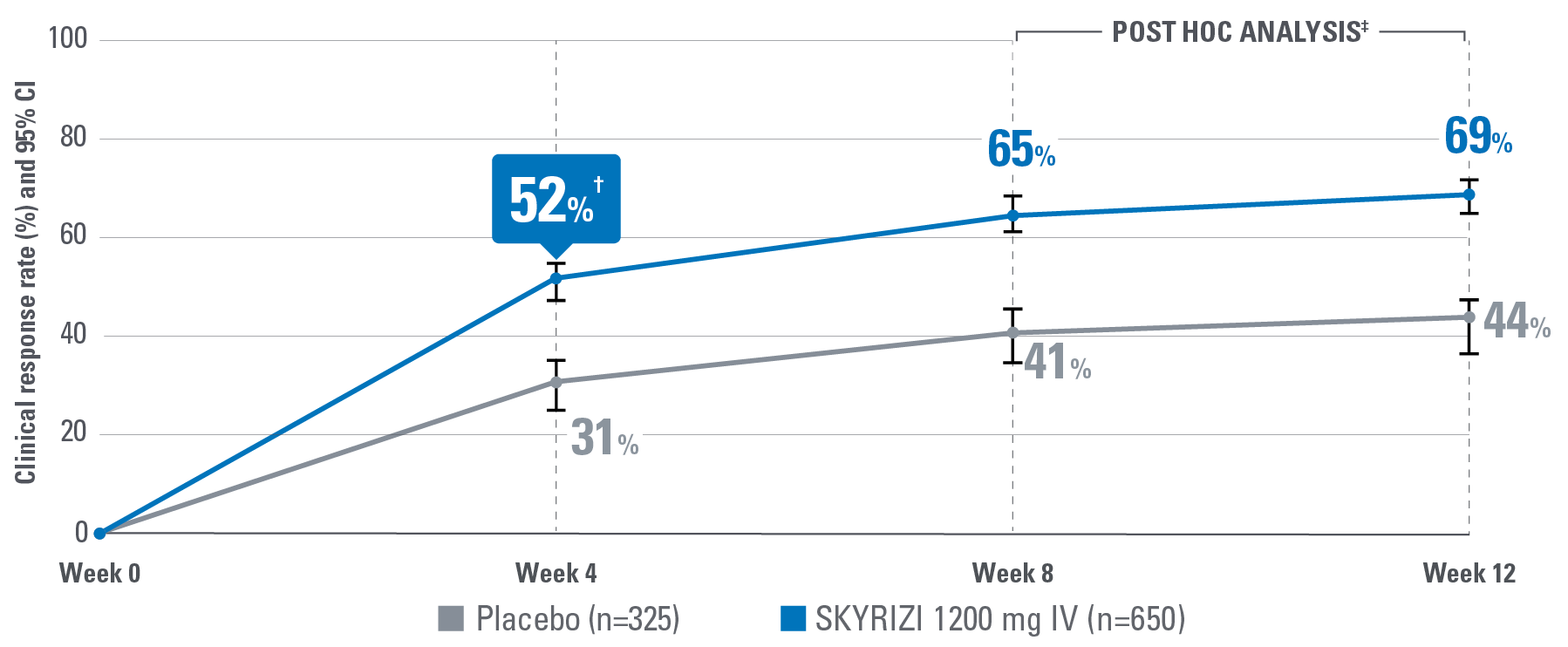
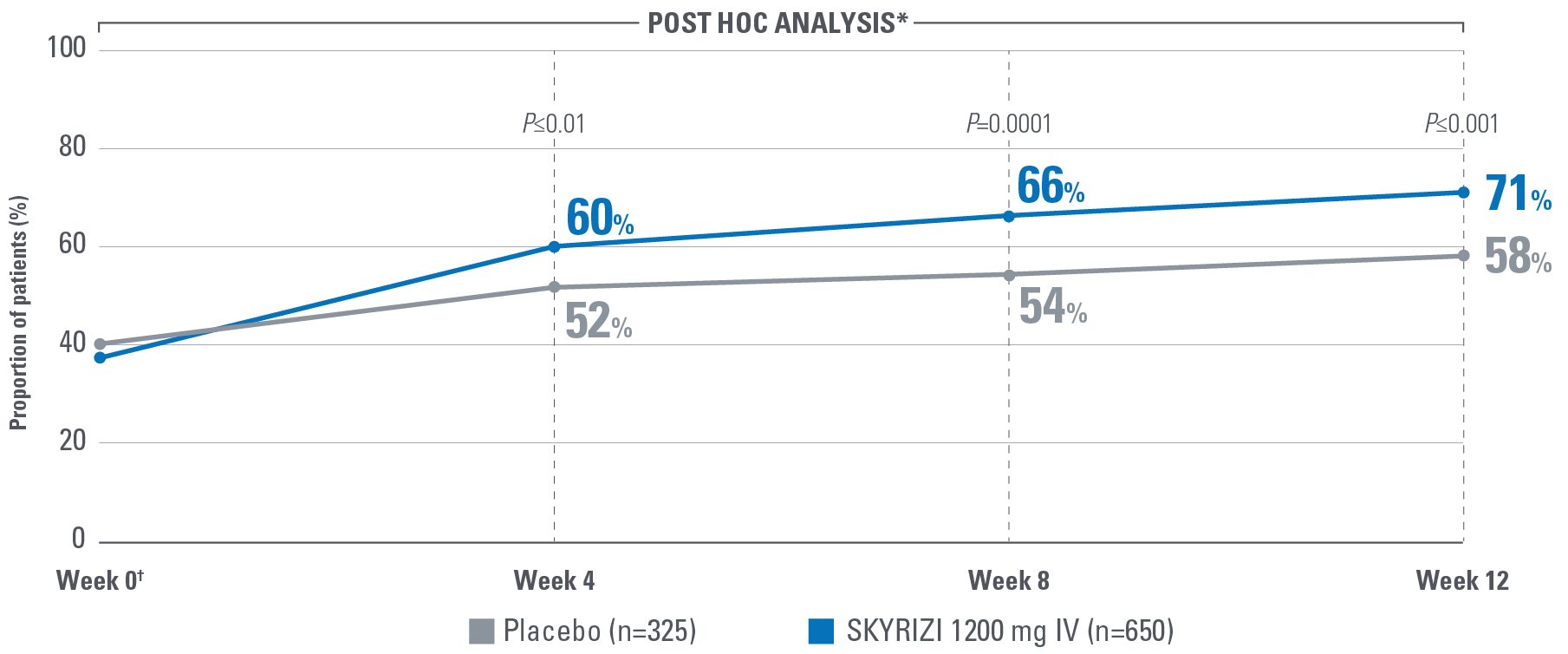
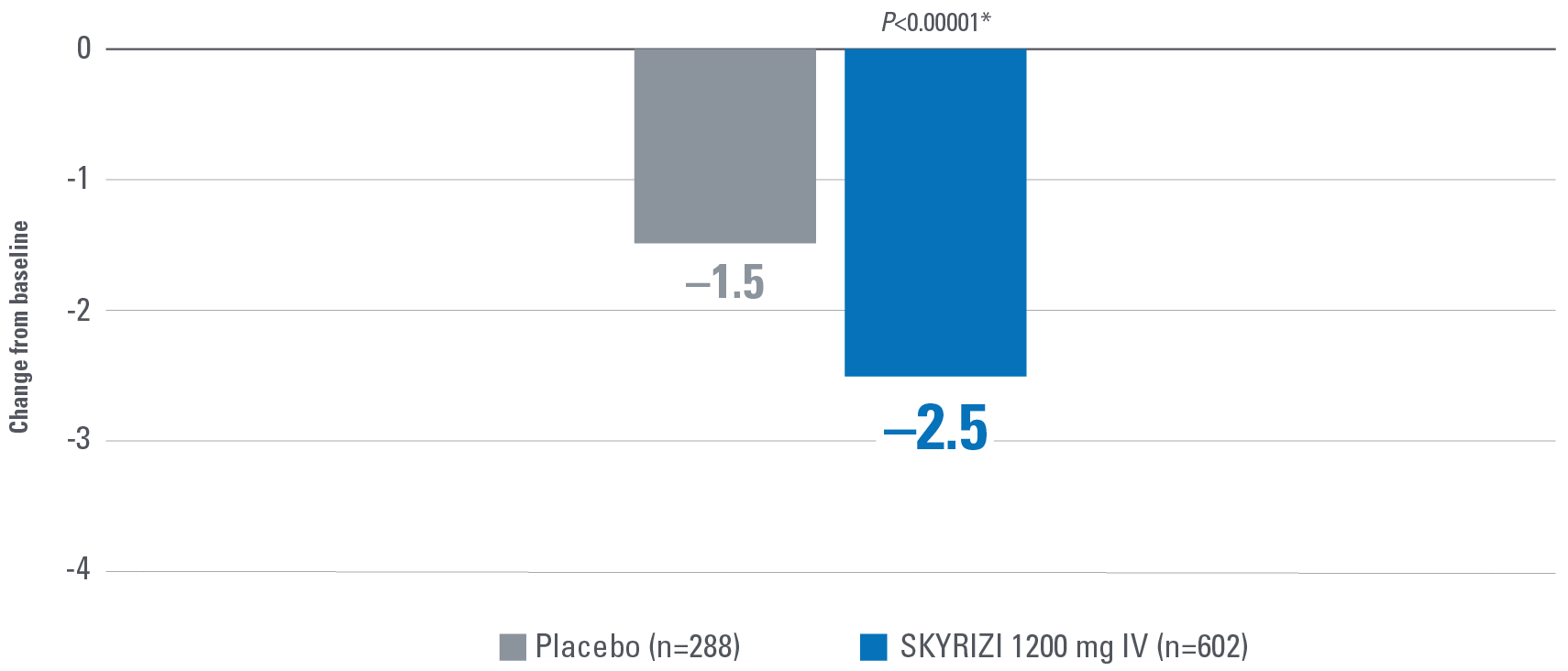
EARLY* CLINICAL RESPONSE AT WEEK 4 OF INDUCTION PER PARTIAL ADAPTED MAYO SCORE (paMS)1
Composed of SFS and RBS
Error bars represent 95% confidence interval.2
*Early is defined as clinical response per paMS achieved at Week 4.1
†Statistically significant under multiplicity-control for SKYRIZI vs placebo comparison (P≤0.00001).1,2
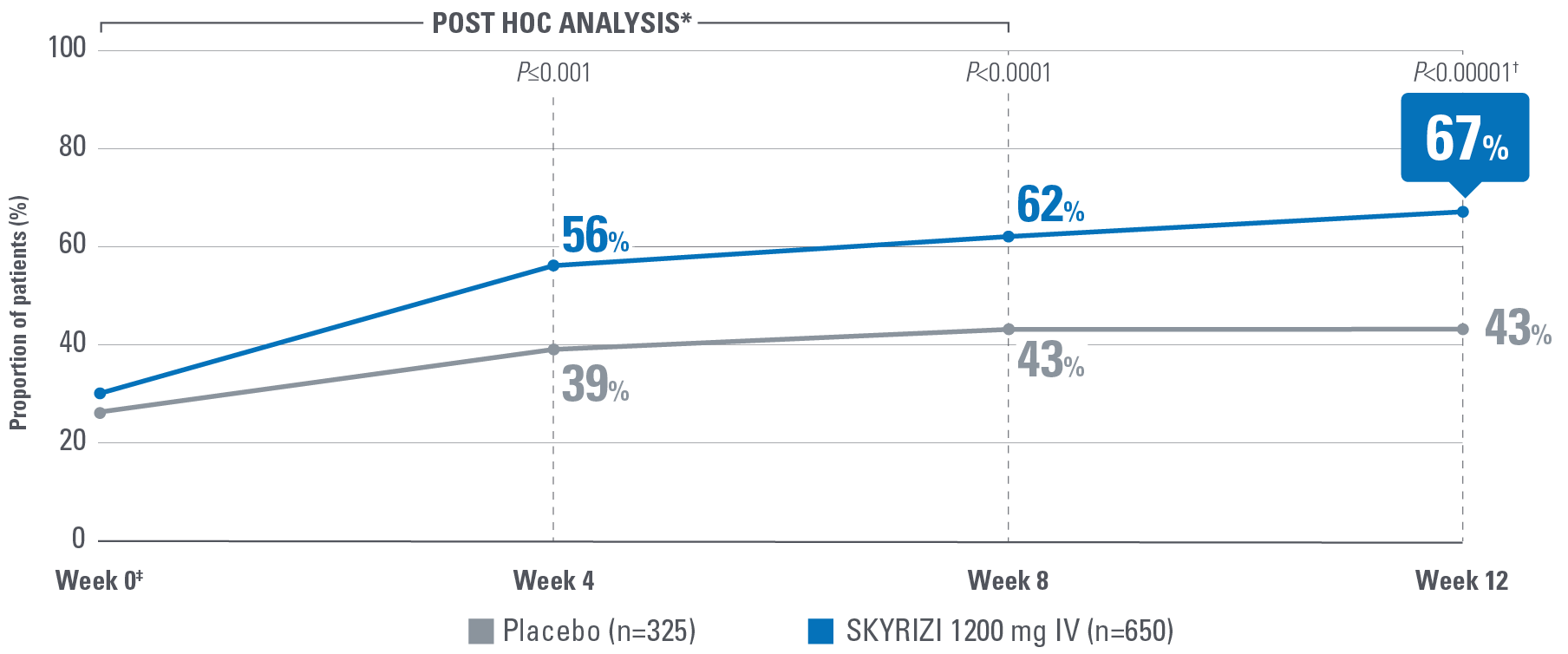
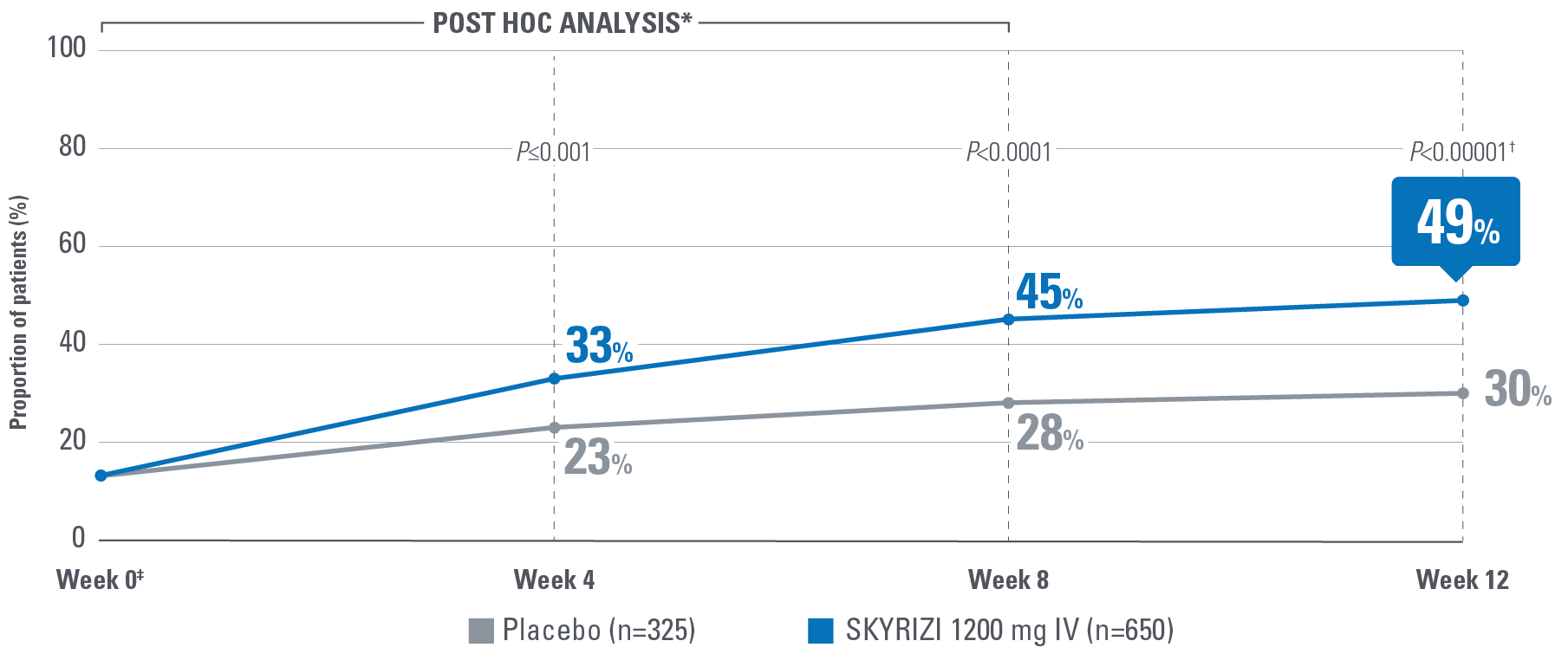
‡POST HOC LIMITATIONS: Post hoc analyses of data are taken from completed clinical trials. All endpoints analyzed in a post hoc analysis were not prespecified in the original trial and are not multiplicity controlled. These data are hypothesis-generating only; no clinical or statistical conclusions can be drawn.
Endpoint definition
Clinical response per paMS: A decrease of ≥1 point and ≥30% from baseline and a decrease in RBS ≥1 or an absolute RBS ≤1.1
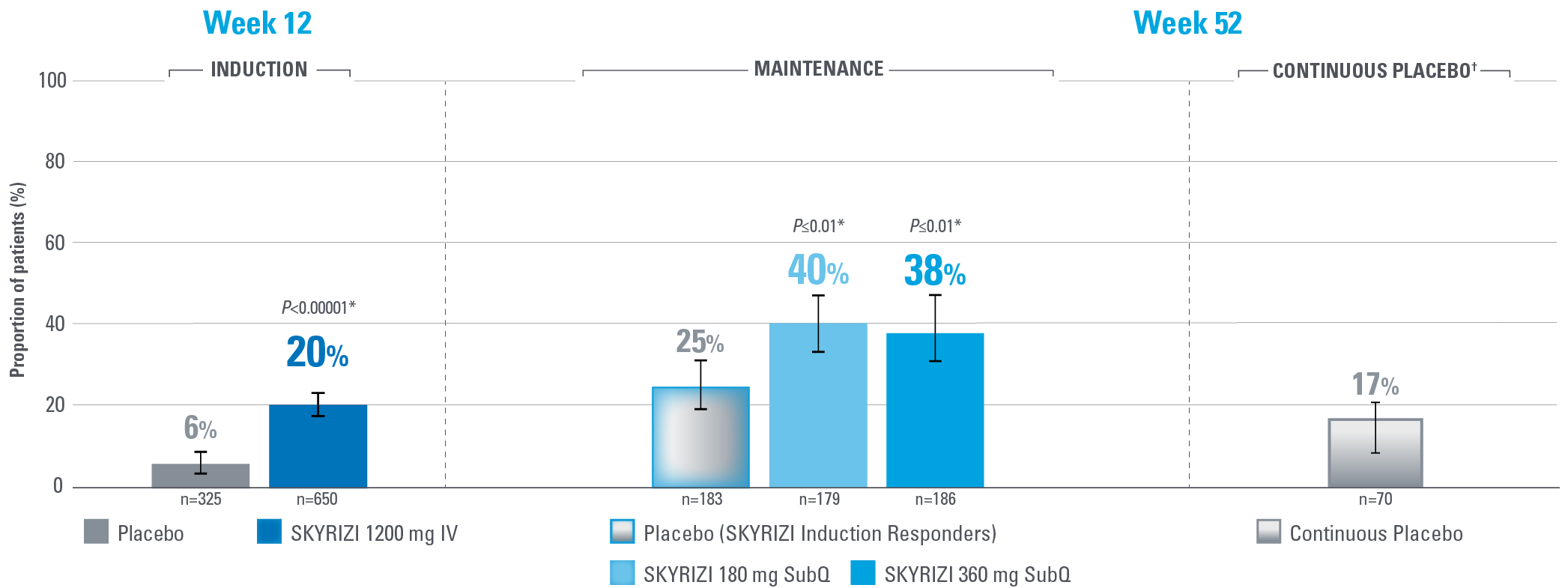
SIGNIFICANT DURABLE CLINICAL REMISSION PER mMS AT WEEKS 12 AND 521,2
Composite of RBS, SFS, and ES
Error bars represent 95% confidence interval.2
*Statistically significant under multiplicity-control for SKYRIZI vs placebo comparison.1,2
†Patients on placebo who responded to induction continued on placebo in maintenance and were not included in the primary efficacy analysis. Continuous placebo data not intended for direct comparison.2
IMPORTANT CONTEXT ABOUT Placebo (SKYRIZI Induction Responders) The placebo group in the maintenance study COMMAND consisted of subjects who achieved clinical response to risankizumab induction therapy and were randomized to receive placebo in the maintenance study (COMMAND). Therefore, the placebo arm is referred to as “Placebo (SKYRIZI Induction Responders)” in the maintenance study sections within this site and referred to as “SKYRIZI IV/Placebo SC” in the SmPC.1
Primary endpoint definition
Clinical remission per mMS: SFS ≤1 and not greater than baseline, RBS=0, and ES ≤1 without evidence of friability.1
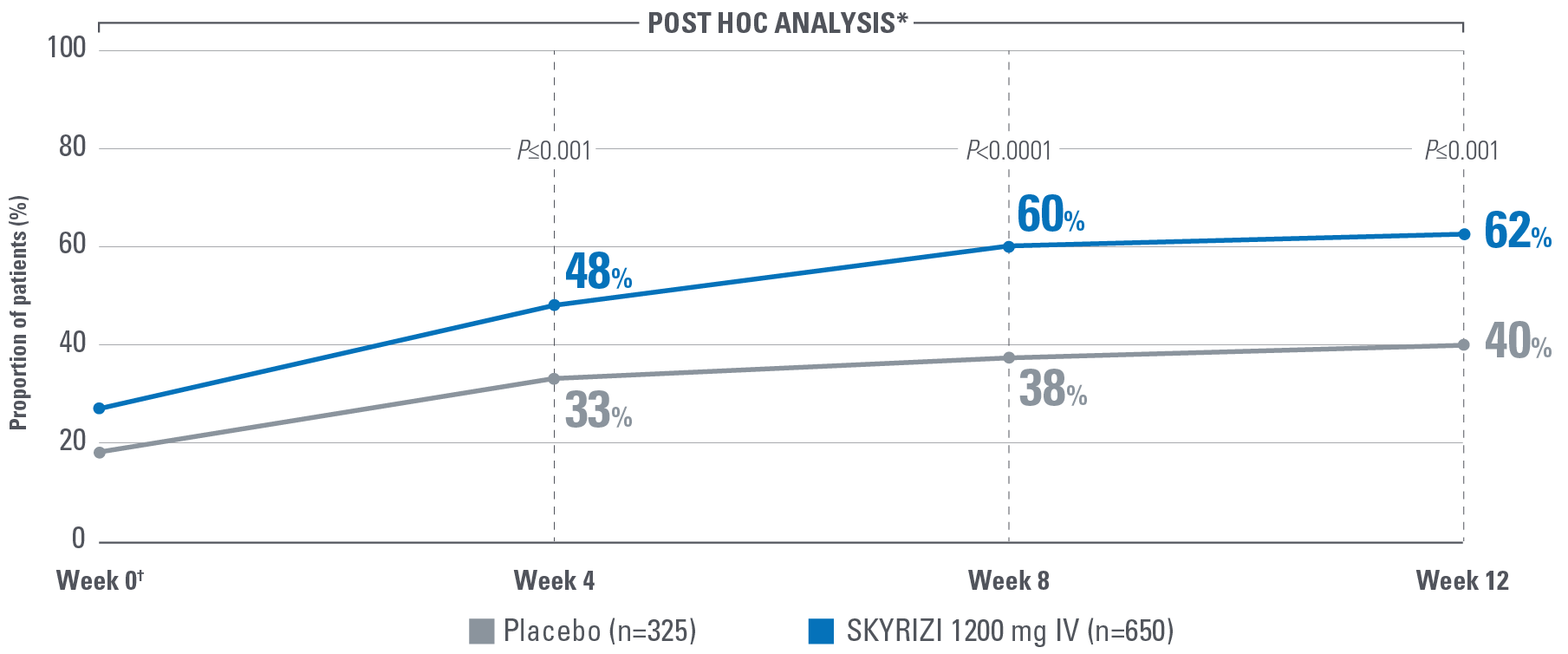
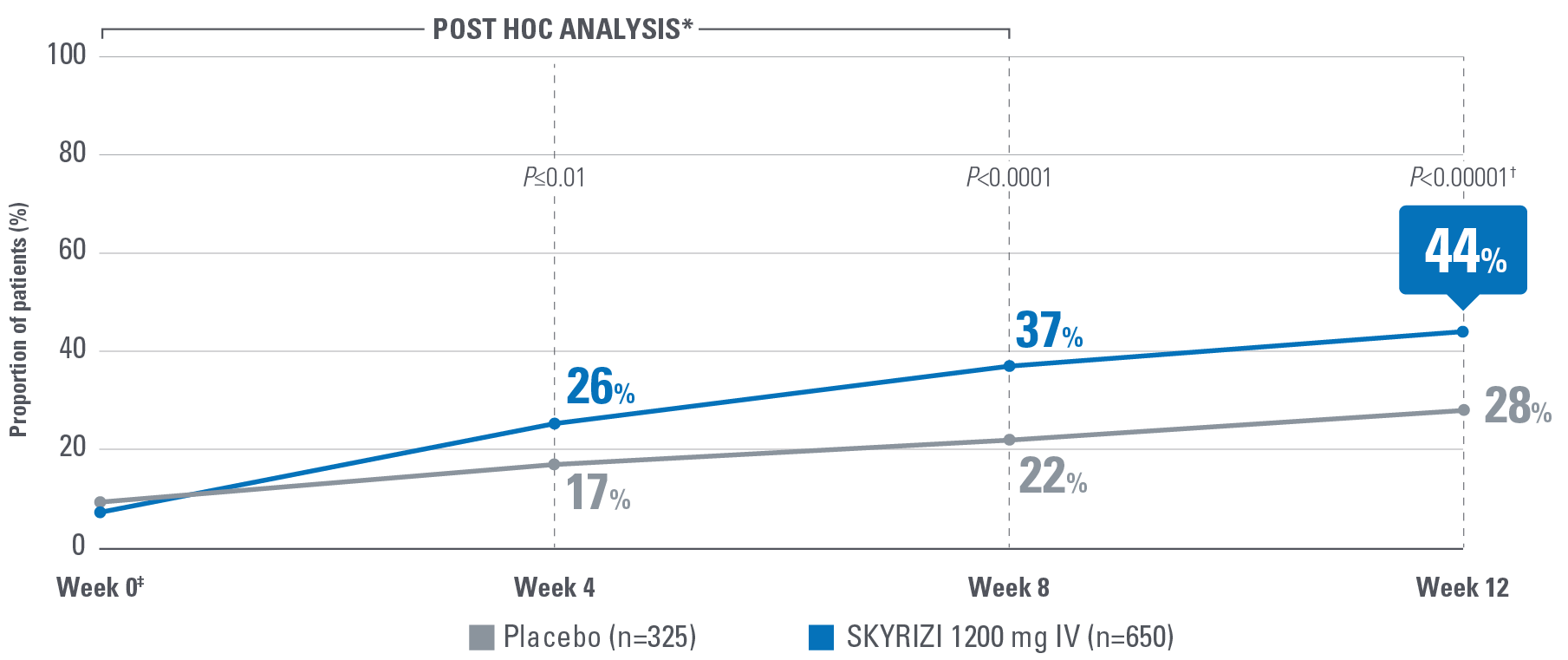
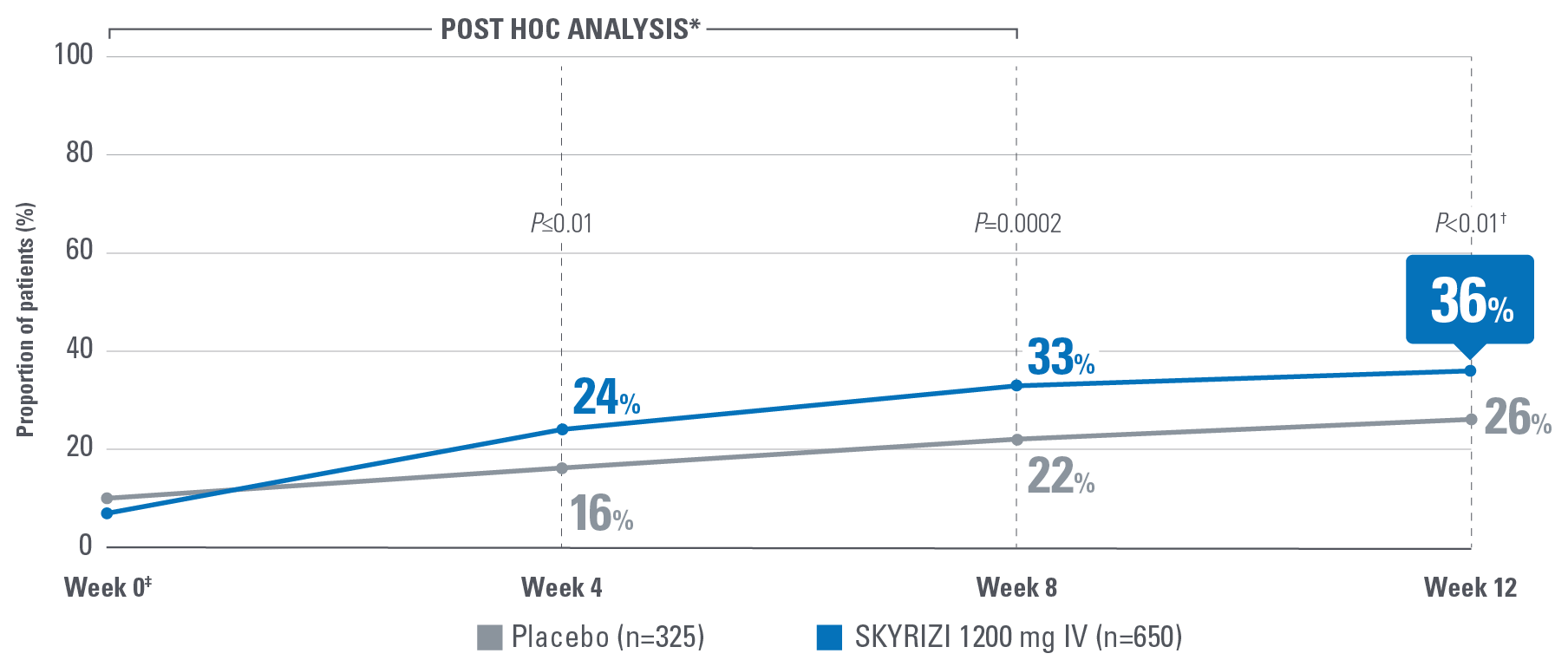
Symptom control is defined by the measurement of 8 patient-reported outcomes, measured at Week 12 and are defined as follows:
- No bowel urgency: A mean score of 0 denoting the absence of symptoms for the 3 most recent days prior to each study visit.2
- No abdominal pain: A mean score of 0 denoting the absence of symptoms for the 3 most recent days prior to each study visit.2
- No nocturnal bowel movements: A mean score of 0 denoting the absence of symptoms for the 3 most recent days prior to each study visit.2
- No tenesmus: A mean score of 0 denoting the absence of symptoms for the 3 most recent days prior to each study visit.2
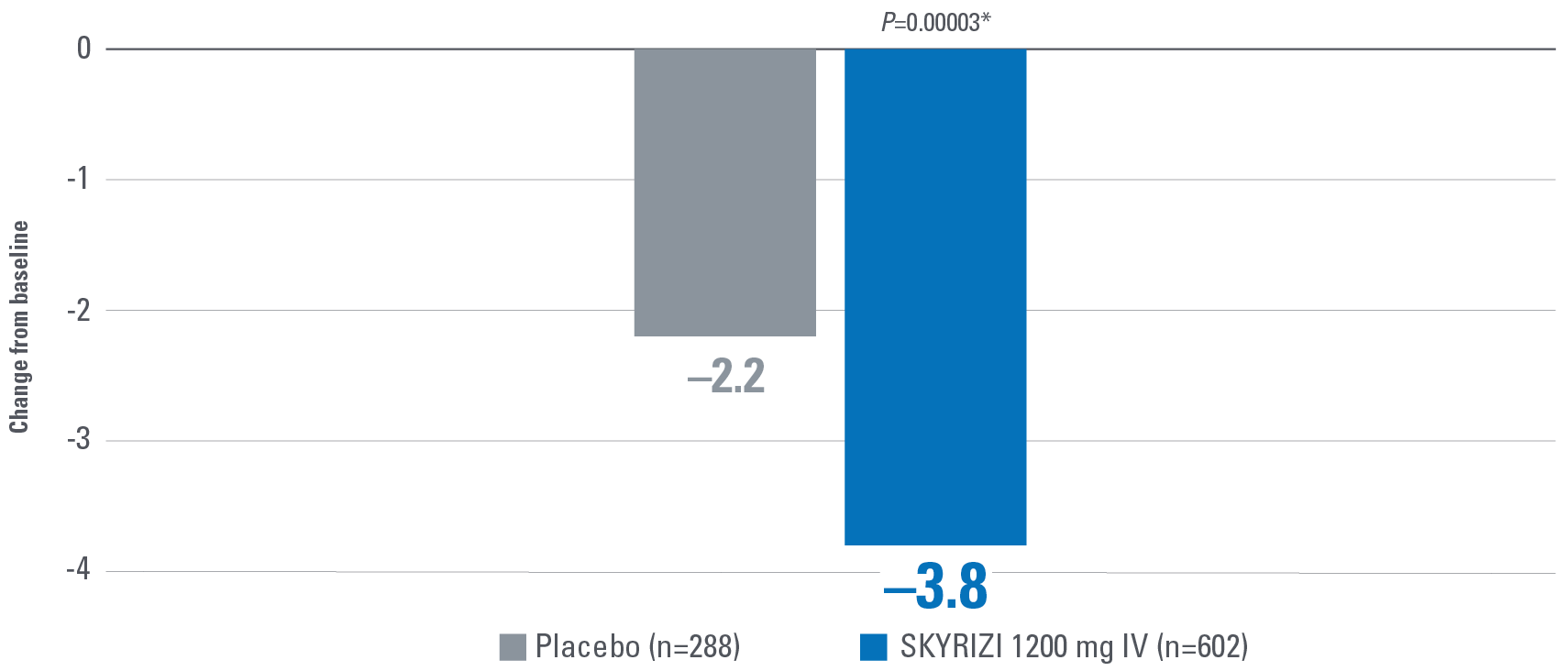
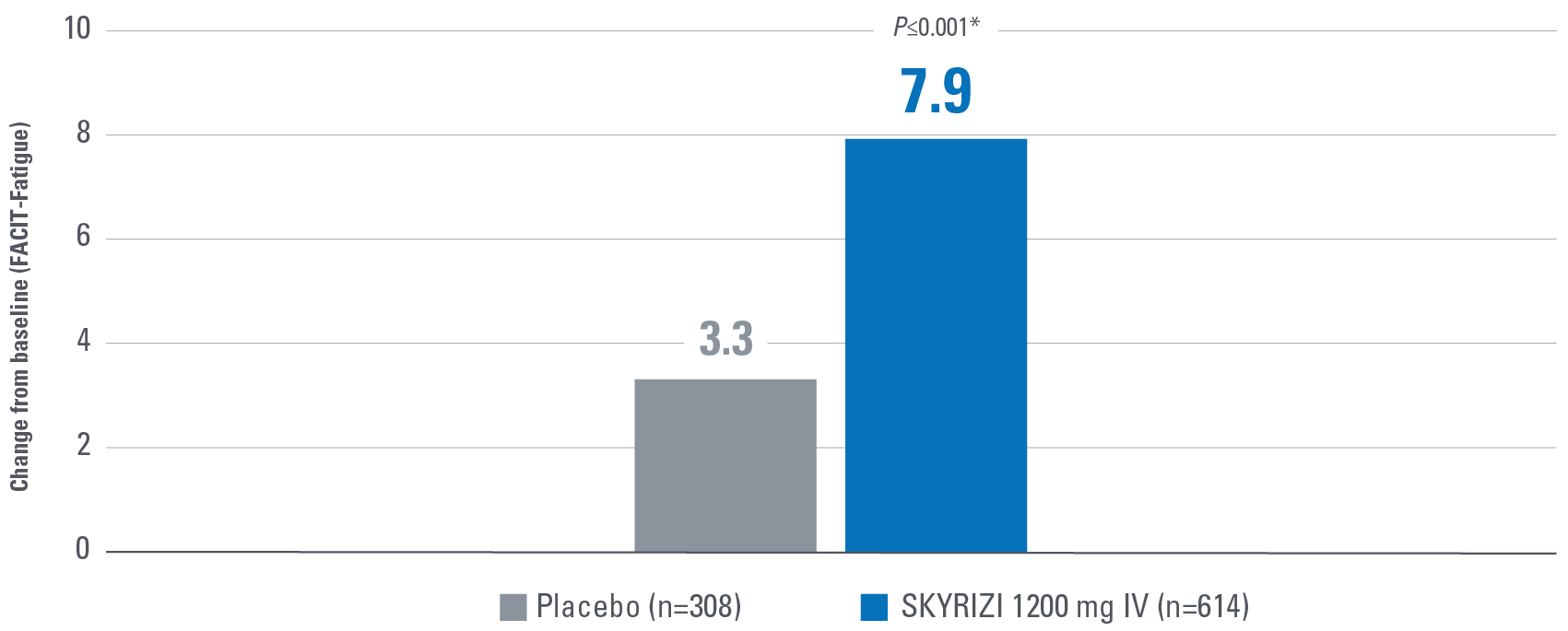
- Change from baseline in Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-Fatigue): A 13-item assessment of fatigue associated with disease and how it impacts daily activities and function. Lower scores indicate greater fatigue.2
- Change from baseline in sleep interruption due to ulcerative colitis symptoms.2
- Change from baseline in fecal incontinence due to ulcerative colitis symptoms.2
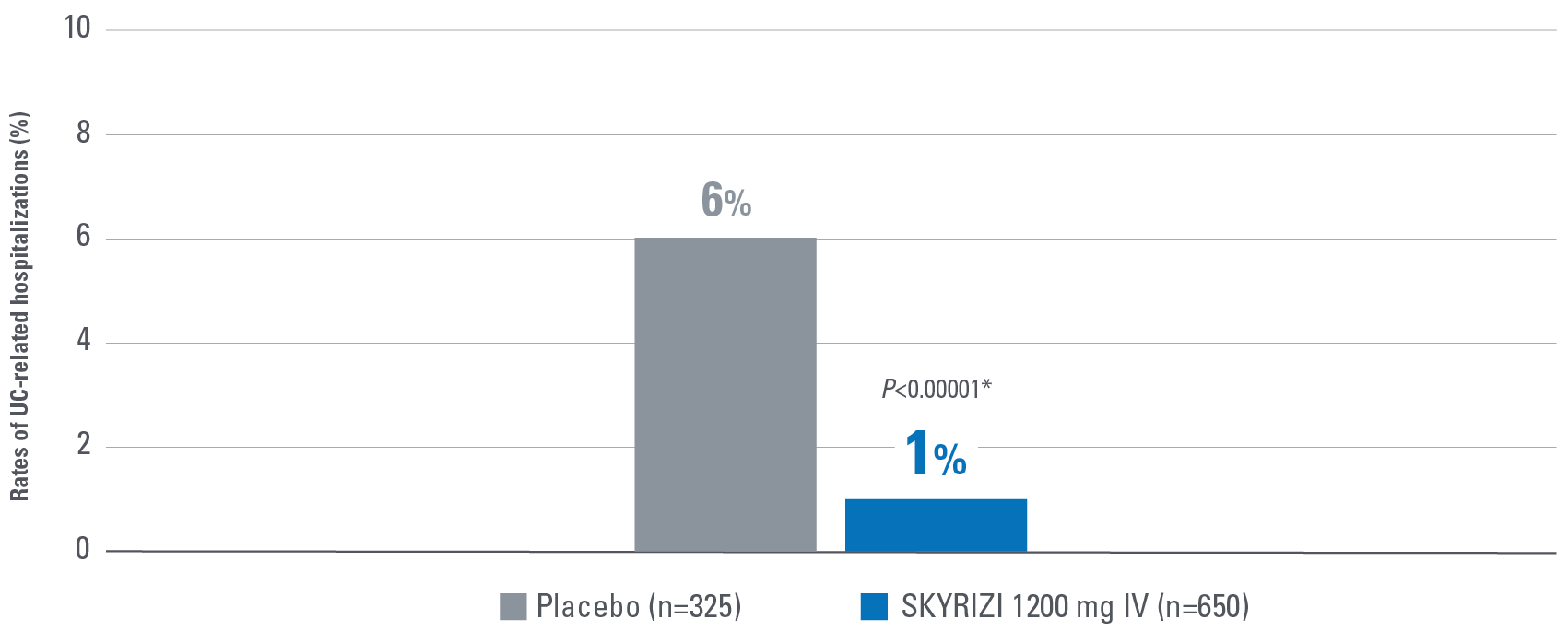
- Hospitalization rates are measures as related to UC symptoms.1,2
Control is defined by the primary endpoint of clinical remission per mMS (INSPIRE: 20% [1200 mg IV]; COMMAND: 40% [180 mg SubQ], 38% [360 mg SubQ]) based on SFS, RBS, and ES at Weeks 12 and 52.1
Indication1
SKYRIZI is indicated for the treatment of adult patients with moderately to severely active ulcerative colitis who have had an inadequate response to, lost response to, or were intolerant to conventional therapy or a biologic therapy.
Important Safety Information1
Affiliate to insert local ISI.
Risankizumab is contraindicated in patients hypersensitive to the active substance or to any of the excipients, and in patients with clinically important active infections (e.g. active tuberculosis).
Risankizumab may increase the risk of infection. In patients with a chronic infection, a history of recurrent infection, or known risk factors for infection, risankizumab should be used with caution. Treatment with risankizumab should not be initiated in patients with any clinically important active infection until the infection resolves or is adequately treated.
Patients treated with risankizumab should be instructed to seek medical advice if signs or symptoms of clinically important chronic or acute infection occur. If a patient develops such an infection or is not responding to standard therapy for the infection, the patient should be closely monitored and risankizumab should not be administered until the infection resolves.
Prior to initiating treatment with risankizumab, patients should be evaluated for tuberculosis (TB) infection. Patients receiving risankizumab should be monitored for signs and symptoms of active TB. Anti-TB therapy should be considered prior to initiating risankizumab in patients with a past history of latent or active TB in whom an adequate course of treatment cannot be confirmed.
Prior to initiating therapy with risankizumab, completion of all appropriate immunisations should be considered according to current immunisation guidelines. If a patient has received live vaccination (viral or bacterial), it is recommended to wait at least 4 weeks prior to starting treatment with risankizumab. Patients treated with risankizumab should not receive live vaccines during treatment and for at least 21 weeks after treatment.
Serious hypersensitivity reactions, including anaphylaxis, have been reported with use of risankizumab. If a serious hypersensitivity reaction occurs, administration of risankizumab should be discontinued immediately and appropriate therapy initiated.
The most frequently reported adverse reactions were upper respiratory infections (13% in psoriasis, 15.6% in Crohn’s disease and 26.2% in ulcerative colitis).
Commonly (≥ 1/100 to < 1/10) reported adverse reactions included tinea infections, headache, pruritus, rash, eczema, fatigue, and injection site reactions.
This is not a complete summary of all safety information.
See SKYRIZI full Summary of Product Characteristics (SmPC) at www.ema.europa.eu
Globally, prescribing information varies; refer to the individual country product label for complete information.
AT failure: advanced therapy failure; AT naive: advanced therapy naive; ES: endoscopic subscore; FACIT-Fatigue: Functional Assessment of Chronic Illness Therapy-Fatigue; HEMH: histologic-endoscopic mucosal healing; IV: intravenous; JAK: Janus kinase; mMS: modified Mayo score; paMS: partial adapted Mayo score; RBS: rectal bleeding subscore; SC: subcutaneous; SFS: stool frequency subscore; SubQ: subcutaneous; S1P: sphingosine 1-phosphate; UC: ulcerative colitis.
References: 1. SKYRIZI [Summary of Product Characteristics]. AbbVie Deutschland GmbH & Co. KG; [DRAFT]. 2. Louis E, Schreiber S, Panaccione R, et al. Risankizumab as ulcerative colitis induction and maintenance therapy: INSPIRE and COMMAND studies. A primary analysis of phase 2b/3 randomized clinical trials. DRAFT. 3. Torres J, Panés J, Siegel CA, et al. Individual and comprehensive symptom resolution after induction and maintenance therapy with risankizumab in patients with moderately to severely active ulcerative colitis: a post hoc analysis of INSPIRE and COMMAND studies. Oral presentation at: 19th Congress of the European Crohn’s and Colitis Organisation; February 21-24, 2024; Stockholm, Sweden. 4. Data on file, AbbVie Inc. ABVRRTI78081.