WELL-ESTABLISHED
SAFETY PROFILE
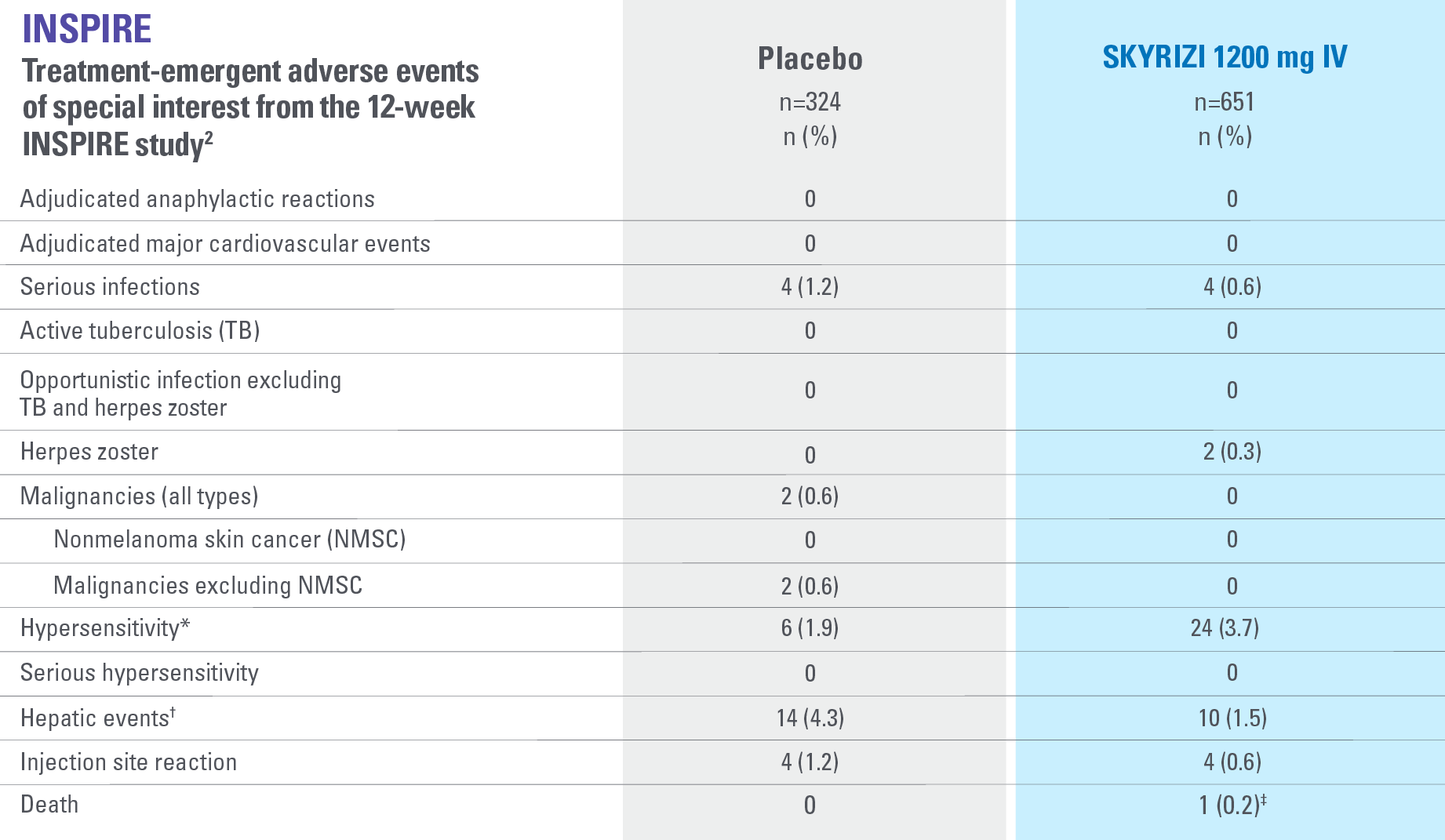
*Events identified with Hypersensitivity SMQ, a broader medical concept than injection and infusion site reactions, includes injection and infusion site-related terms, i.e., injection site rash, which overlap with the term injection site reaction CMQ. Hypersensitivity includes both nonserious and serious hypersensitivity reaction events.2
†All hepatic events were identified with search criteria covering the standardized MedDRA queries of hepatic failure, fibrosis and cirrhosis and other liver damage-related conditions, hepatitis, noninfectious, cholestasis and jaundice of hepatic origin, liver-related investigations, signs and symptoms, and liver-related coagulation and bleeding disturbances.2
‡The death was related to COVID-19 (pneumonia).2
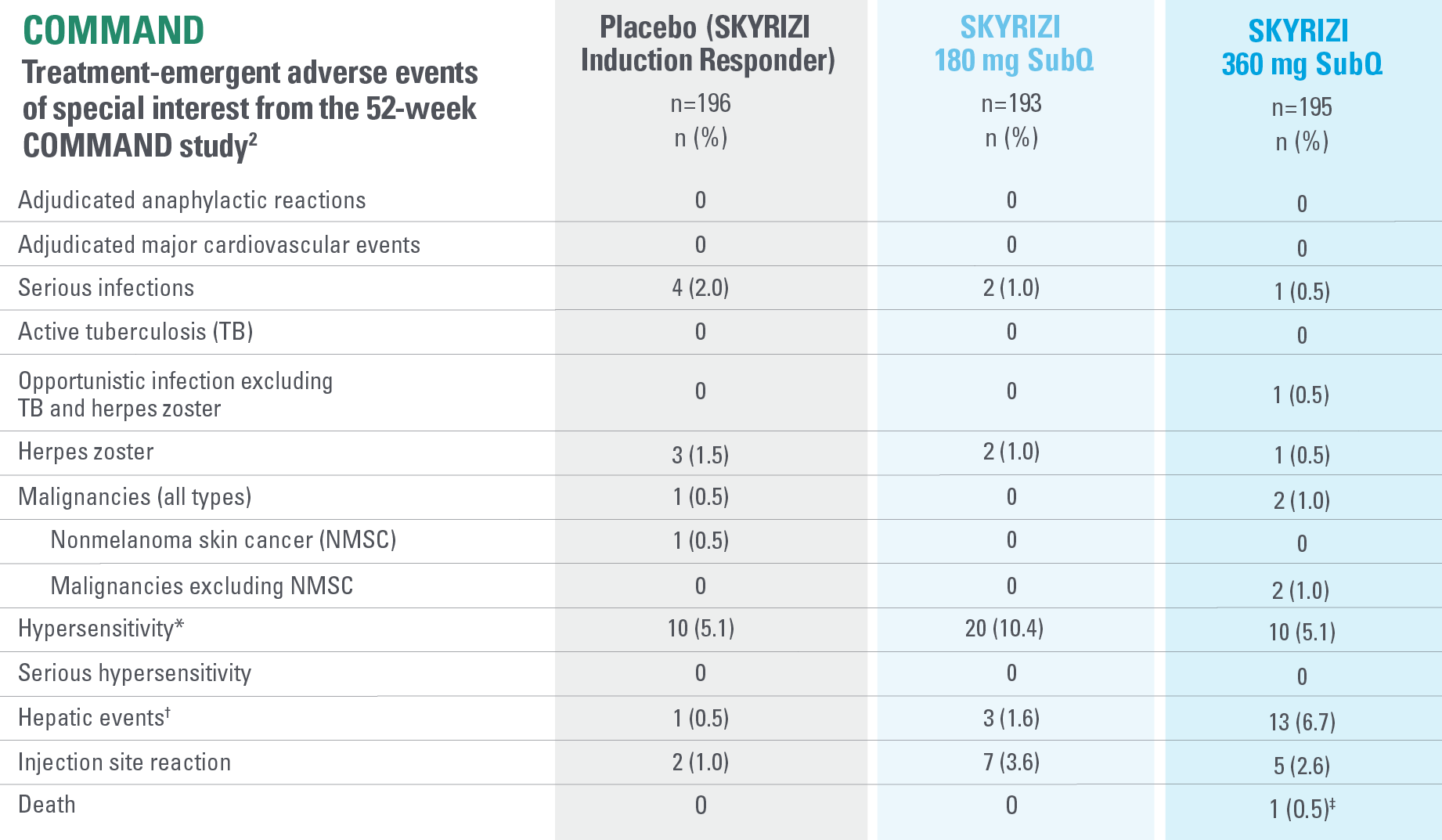
*Events identified with Hypersensitivity SMQ, a broader medical concept than injection and infusion site reactions, includes injection and infusion site-related terms, i.e., injection site rash, which overlap with the term injection site reaction CMQ. Hypersensitivity includes both nonserious and serious hypersensitivity reaction events.2
†All hepatic events were identified with search criteria covering the standardized MedDRA queries of hepatic failure, fibrosis and cirrhosis and other liver damage-related conditions, hepatitis, noninfectious, cholestasis and jaundice of hepatic origin, liver-related investigations, signs and symptoms, and liver-related coagulation and bleeding disturbances.2
‡One death was reported in a patient treated with risankizumab 360 mg who had a diagnosis of colon adenocarcinoma. The death of the patient was due to the colon adenocarcinoma and found to have no reasonable possibility of being related to study drug.2
IMPORTANT CONTEXT ABOUT Placebo (SKYRIZI induction responders): The placebo group in the maintenance study COMMAND consisted of subjects who achieved clinical response to risankizumab induction therapy and were randomized to receive placebo in the maintenance study (COMMAND). Therefore, the placebo arm is referred to as “Placebo (SKYRIZI Induction Responders)” in the maintenance study sections within this site and referred to as “SKYRIZI IV/Placebo SC” in the SmPC.1
SAFETY FOR SKYRIZI IN UC AT WEEK 52 WAS EVALUATED IN:
388
Patients across treatment arms2
358.9
Patient-years of exposure2
As of 2023.
THERAPEUTIC AREAS
Plaque psoriasis
SKYRIZI is indicated for the treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic therapy.1
Psoriatic arthritis
SKYRIZI, alone or in combination with methotrexate (MTX), is indicated for the treatment of active psoriatic arthritis in adults who have had an inadequate response or who have been intolerant to one or more disease-modifying antirheumatic drugs (DMARDs).1
Ulcerative colitis
SKYRIZI is indicated for the treatment of adult patients with moderately to severely active ulcerative colitis who have had an inadequate response to, lost response to, or were intolerant to conventional, or biologic, or JAK inhibitor therapy.1
Crohn’s disease
SKYRIZI is indicated for the treatment of adult patients with moderately to severely active Crohn’s disease who have had an inadequate response to, lost response to, or were intolerant to conventional therapy or a biologic therapy.1
*Includes 2 UC Phase 3 studies (INSPIRE and COMMAND). Includes 5 CD Phase 1-3 studies (including ADVANCE, MOTIVATE, and FORTIFY). Includes 4 PsA Phase 1-3 studies (including KEEPsAKE-1 and KEEPsAKE-2). Includes 17 Phase 1-3 studies in PsO encompassing 5 trials using integrated data evaluated at Week 16 (including UltIMMa-1, UltIMMa-2, IMMhance, and IMMVent), and 12 additional trials including LIMMitless.1-7
†Source: OUS Information derived from MySource Data affiliates tracking. US information derived from Symphony Health (PatientSource), IQVIA (NPA), and SKYRIZI Bridge Program data using proprietary methodology (up to December 2023).8
‡Safety data were evaluated for all patients receiving >1 dose of SKYRIZI from Phase 1-3 trials, including open-label extension and dose ranging studies.1,2
§CD: SKYRIZI 600 mg, SKYRIZI 360 mg, risankizumab 1200 mg, risankizumab 200 mg, and risankizumab 180 mg (risankizumab 1200 mg, 200 mg, and 180 mg are not EMA-approved doses in CD); UC: SKYRIZI 1200 mg, SKYRIZI 180 mg, SKYRIZI 360 mg, risankizumab 1800 mg, risankizumab 600 mg (risankizumab 1800 mg and 600 mg are not approved doses in UC); PsA: SKYRIZI 150 mg; PsO: all administered doses ranging from 18 mg to 180 mg of risankizumab including SKYRIZI 150 mg (the only dose on label for PsO is 150 mg).1,3
Information presented on this page as of February 2024.
Indication1
SKYRIZI is indicated for the treatment of adult patients with moderately to severely active ulcerative colitis who have had an inadequate response to, lost response to, or were intolerant to conventional therapy or a biologic therapy.
Important Safety Information1
Affiliate to insert local ISI.
Risankizumab is contraindicated in patients hypersensitive to the active substance or to any of the excipients, and in patients with clinically important active infections (e.g. active tuberculosis).
Risankizumab may increase the risk of infection. In patients with a chronic infection, a history of recurrent infection, or known risk factors for infection, risankizumab should be used with caution. Treatment with risankizumab should not be initiated in patients with any clinically important active infection until the infection resolves or is adequately treated.
Patients treated with risankizumab should be instructed to seek medical advice if signs or symptoms of clinically important chronic or acute infection occur. If a patient develops such an infection or is not responding to standard therapy for the infection, the patient should be closely monitored and risankizumab should not be administered until the infection resolves.
Prior to initiating treatment with risankizumab, patients should be evaluated for tuberculosis (TB) infection. Patients receiving risankizumab should be monitored for signs and symptoms of active TB. Anti-TB therapy should be considered prior to initiating risankizumab in patients with a past history of latent or active TB in whom an adequate course of treatment cannot be confirmed.
Prior to initiating therapy with risankizumab, completion of all appropriate immunisations should be considered according to current immunisation guidelines. If a patient has received live vaccination (viral or bacterial), it is recommended to wait at least 4 weeks prior to starting treatment with risankizumab. Patients treated with risankizumab should not receive live vaccines during treatment and for at least 21 weeks after treatment.
Serious hypersensitivity reactions, including anaphylaxis, have been reported with use of risankizumab. If a serious hypersensitivity reaction occurs, administration of risankizumab should be discontinued immediately and appropriate therapy initiated.
The most frequently reported adverse reactions were upper respiratory infections (13% in psoriasis, 15.6% in Crohn’s disease and 26.2% in ulcerative colitis).
Commonly (≥ 1/100 to < 1/10) reported adverse reactions included tinea infections, headache, pruritus, rash, eczema, fatigue, and injection site reactions.
This is not a complete summary of all safety information.
See SKYRIZI full Summary of Product Characteristics (SmPC) at www.ema.europa.eu
Globally, prescribing information varies; refer to the individual country product label for complete information.
CD: Crohn’s disease; CMQ: customized MedDRA query; COVID-19: coronavirus disease 2019; IV: intravenous; JAK: Janus kinase; MedDRA: Medical Dictionary for Regulatory Activities; NMSC: nonmelanoma skin cancer; OUS: outside US; PsA: psoriatic arthritis; PsO: plaque psoriasis; PYs: patient-years; SMQ: standardized MedDRA query; SubQ: subcutaneous; TB: tuberculosis.
References: 1. SKYRIZI [Summary of Product Characteristics]. AbbVie Deutschland GmbH & Co. KG; [DRAFT]. 2. Louis E, Schreiber S, Panaccione R, et al. Risankizumab as ulcerative colitis induction and maintenance therapy: INSPIRE and COMMAND studies. A primary analysis of phase 2b/3 randomized clinical trials. DRAFT. 3. Gordon KB, Blauvelt A, Bachelez H, et al. Long-term safety of risankizumab in patients with psoriatic disease: findings from integrated analyses of 17 clinical trials in psoriasis and 4 in psoriatic arthritis. Poster presented at: European Congress of Rheumatology; June 1-4, 2022; Copenhagen, Denmark. 4. D’Haens G, Panaccione R, Baert F, et al. Risankizumab as induction therapy for Crohn’s disease: results from the phase 3 ADVANCE and MOTIVATE induction trials. Lancet. 2022;399(10340):2015-2030. doi:10.1016/S0140-6736(22)00467-6 5. Ferrante M, Panaccione R, Baert F, et al. Risankizumab as maintenance therapy for moderately to severely active Crohn’s disease: results from the multicentre, randomised, double-blind, placebo-controlled, withdrawal phase 3 FORTIFY maintenance trial. Lancet. 2022;399(10340):2031-2046. doi:10.1016/S0140-6736(22)00466-4 6. Feagan BG, Sandborn WJ, D’Haens G, et al. Induction therapy with the selective interleukin-23 inhibitor risankizumab in patients with moderate-to-severe Crohn’s disease: a randomised, double-blind, placebo-controlled phase 2 study. Lancet. 2017;389(10080):1699-1709. doi:10.1016/S0140-6736(17)30570-6 7. Feagan BG, Panés J, Ferrante M, et al. Risankizumab in patients with moderate to severe Crohn’s disease: an open-label extension study. Lancet Gastroenterol Hepatol. 2018;3(10):671-680. doi:10.1016/S2468-1253(18)30233-4 8. Data on file. ABVRRT177562. 9. Data on file. ABVRRT173682.