SKYRIZI (risankizumab) is an IL-23/p19 inhibitor indicated for the treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic therapy. SKYRIZI, alone or in combination with MTX, is also indicated for the treatment of active psoriatic arthritis in adults who have had an inadequate response or who have been intolerant to one or more DMARDs.1
SKYRIZI: DEMONSTRATED SUPERIORITY
at achieving complete skin clearance in PsO versus 3 agents in different biologic classes1,4,11-13
Durable, complete clearance
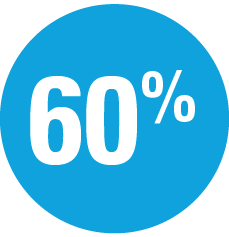
Superior to ustekinumab
at Week 16 and Week 52: UltIMMa pivotal trials (P<0.001)1,4
- 2x the percentage of patients achieved PASI 100 at Week 16 and Week 52 with SKYRIZI (UltIMMa-2)
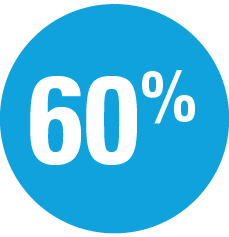
Superior to secukinumab
at Week 52: IMMerge assessor-blinded Phase 3b trial (P<0.001)11,12
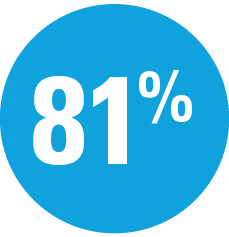
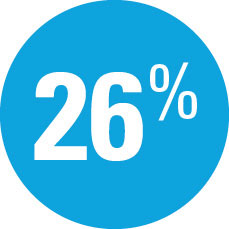
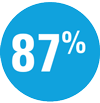
Among adalimumab intermediate responders (PASI 50 to <PASI 90) who were rerandomized at Week 16:
| — | Week 44: 40% SKYRIZI vs 7% adalimumab achieved PASI 100 |
| — | Week 44: 66% SKYRIZI vs 21% adalimumab achieved PASI 90† |
| * | Co-primary endpoint. Note: Some studies were designed with primary endpoints in two parts (Part A and Part B), per protocol. All other endpoints were ranked secondary. |
| † | Primary endpoint. All other endpoints were ranked secondary. |
NO DOSE ADJUSTMENT REQUIRED, regardless of baseline characteristics, including BMI and weight1,14,15†
- 1 injection/dose for both the SKYRIZI prefilled pen and syringe
- SKYRIZI is dosed 150 mg (one 150-mg subcutaneous injection) at Week 0, Week 4, and every 12 weeks thereafter for adult patients with moderate to severe PsO or active PsA1
- Consideration should be given to discontinuing treatment in patients who have shown no response after 16 weeks of treatment. Some plaque psoriasis patients with initial partial response may subsequently improve with continued treatment beyond 16 weeks.
| * | Maintenance dosing (one 150-mg subcutaneous injection/dose) every 12 weeks following a starter dose at Week 0 and Week 4. |
| † | Numeric trends toward less efficacy were observed in clinical trials in patients weighing more than 130 kg. However, this observation is based on a limited number of subjects. |
- PsO safety profile similar to ustekinumab through Week 52 during RCTs1,4
- Studied in >3,650 PsO patients across >13,300 PYs in an OLE16
- Studied in >1,500 PsA patients across >3,800 PYs in an OLE16
PsA safety profile consistent with the safety profile observed in PsO1
| * | Integrated all-risankizumab safety data sets (data cutoff March 25, 2023) from 20 Phase 1–4 clinical trials in PsO and 4 Phase 2 and 3 trials in PsA. Median (range) of treatment duration for PsO was 4.1 years (81 days to 8.8 years) and for PsA was 2.8 years (84 days to 4.0 years).16 |
UltIMMa-1 and UltIMMa-2 replicate Phase 3 study designs1,4
UltIMMa-1/2 were two replicate 52-week, randomized, placebo-controlled comparative studies of SKYRIZI vs ustekinumab in adult patients with moderate to severe chronic plaque psoriasis.1,4
Data analysis: Missing data were imputed as nonresponders (NRI) for categorical endpoints and by last observation carried forward for continuous endpoints.
Co-primary endpoints: Proportion of patients who achieved PASI 90 response and an sPGA score of clear or almost clear (sPGA 0 or 1) at Week 16 vs placebo.
Ranked secondary endpoints: All 15 ranked secondary endpoints vs placebo and/or ustekinumab at Week 16 and/or Week 52 were met in both UltIMMa-1 and UltIMMa-2 (P<0.0001). PASI 90 and PASI 100 clearance of psoriatic lesions at Week 52 vs ustekinumab were ranked secondary endpoints.
Dosing: SKYRIZI 150 mg (two 75-mg subcutaneous injections) at Week 0, Week 4, and every 12 weeks thereafter. Ustekinumab 45 mg or 90 mg (weight-based per label). Ustekinumab was dosed every 12 weeks after 2 starter doses at Week 0 and Week 4.
IMMerge SKYRIZI vs secukinumab Phase 3b study design11,12
A Phase 3b, multicenter, randomized, open-label, efficacy assessor-blinded, active-comparator study designed to evaluate the safety and efficacy of SKYRIZI compared to secukinumab in adult patients with moderate to severe plaque psoriasis.
Patients were randomized 1:1 to SKYRIZI (n=164) (150 mg), given as two 75-mg subcutaneous injections at baseline, 4 weeks later, and every 12 weeks thereafter, or secukinumab (n=163) (300 mg) given as two 150-mg subcutaneous injections, at baseline, Weeks 1, 2, 3, and 4, and then every 4 weeks thereafter.
Safety was assessed in all patients.
Data analysis: Missing data were imputed as nonresponders (NRI) for all primary and ranked secondary endpoints.
Primary endpoints:
• PASI 90 at Week 16 (noninferiority)
• PASI 90 at Week 52 (superiority)
Ranked secondary endpoints:
• PASI 100 at Week 52
• PASI 75 at Week 52
• sPGA 0/1 at Week 52
IMMvent pivotal Phase 3 study design13
A 44-week, randomized comparative study vs adalimumab in adult patients with moderate to severe chronic plaque psoriasis (N=605). IMMvent was powered to show superiority of SKYRIZI over adalimumab in achieving:
Co-primary endpoints:
- PASI 90 at Week 16
- sPGA 0/1 at Week 16
Primary endpoint after rerandomization:
- PASI 90 at Week 44
Ranked secondary endpoints:
- PASI 75 at Week 16
- PASI 100 at Weeks 16 and 44 (rerandomized patients only at Week 44)
Part A (baseline to Week 16): Patients received either SKYRIZI 150 mg or adalimumab (80 mg at baseline, 40 mg at Week 1, and then once every 2 weeks).
Part B (Weeks 16 to 44): The SKYRIZI group continued on treatment. For the adalimumab group, treatment regimen was dependent on the level of PASI response:
- PASI <50 switched to SKYRIZI
- PASI ≥50 to <PASI 90 (rerandomized to either SKYRIZI or adalimumab)
- ≥PASI 90 continued with adalimumab
Adalimumab patients who switched to risankizumab in Part B were dosed at Weeks 16, 20, and 32.
ACR=American College of Rheumatology; ADA=adalimumab; AE=adverse events; bio-IR=inadequate response to a biologic; BMI=body mass index; CI=confidence interval; csDMARD-IR=inadequate response to a conventional synthetic DMARD; DLQI=Dermatology Life Quality Index; DMARD=disease-modifying antirheumatic drug; E=event; IBD=inflammatory bowel disease; IL=interleukin; LDI=Leeds Dactylitis Index; LEI=Leeds Enthesitis Index; MACE=major adverse cardiovascular event; MTX=methotrexate; NMSC=nonmelanoma skin cancer; NRI=nonresponder imputation; OC=observed case; OLE=open-label extension; PASI=Psoriasis Area and Severity Index; PBO=placebo; PsA=psoriatic arthritis; PsO=psoriasis; PSS=psoriasis symptom scale; PY=patient-year; RCT=randomized controlled trial; sPGA=static Physician’s Global Assessment; TB=tuberculosis; TEAE=treatment-emergent adverse event; TNF=tumor necrosis factor; UST=ustekinumab.
EU Indications and Important Safety Information for SKYRIZI
INDICATIONS1
Skyrizi (risankizumab) is indicated for the treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic therapy.
Skyrizi, alone or in combination with methotrexate (MTX), is indicated for the treatment of active psoriatic arthritisin adults who have had an inadequate response or who have been intolerant to one or more disease-modifying antirheumaticdrugs (DMARDs).
Skyrizi is indicated for the treatment of adult patients with moderately to severely active Crohn’s disease who have had an inadequate response to, lost response to, or were intolerant to conventional therapy or a biologic therapy.
IMPORTANT SAFETY INFORMATION1
Risankizumab is contraindicated in patients hypersensitive to the active substance or to any of the excipients, and in patients with clinically important active infections (e.g. active tuberculosis). Risankizumab may increase the risk of infection. In patients with a chronic infection, a history of recurrent infection, or known risk factors for infection, risankizumab should be used with caution. Treatment with risankizumab should not be initiated in patients with any clinically important active infection until the infection resolves or is adequately treated.
Patients treated with risankizumab should be instructed to seek medical advice if signs or symptoms of clinically important chronic or acute infection occur. If a patient develops such an infection or is not responding to standard therapy for the infection, the patient should be closely monitored and risankizumab should not be administered until the infection resolves.
Prior to initiating treatment with risankizumab, patients should be evaluated for tuberculosis (TB) infection. Patients receiving risankizumab should be monitored for signs and symptoms of active TB. Anti-TB therapy should be considered prior to initiating risankizumab in patients with a past history of latent or active TB in whom an adequate course of treatment cannot be confirmed.
Prior to initiating therapy with risankizumab, completion of all appropriate immunisations should be considered according to current immunisation guidelines. If a patient has received live vaccination (viral or bacterial), it is recommended to wait at least 4 weeks prior to starting treatment with risankizumab. Patients treated with risankizumab should not receive live vaccines during treatment and for at least 21 weeks after treatment.
If a serious hypersensitivity reaction occurs, administration of risankizumab should be discontinued immediately and appropriate therapy initiated.
The most frequently reported adverse reactions were upper respiratory infections (from 13% in psoriasis to 15.6% in Crohn’s disease). Commonly (≥ 1/100 to < 1/10) reported adverse reactions included tinea infections, headache, pruritus, rash, fatigue, and injection site reactions.
This is not a complete summary of all safety information. Please see the SmPC for complete prescribing information.
Find out more about SKYRIZI
References
- SKYRIZI [Summary of Product Characteristics]. AbbVie Ltd; March 2024.
- Blome C, Gosau R, Radtke MA, et al. Patient-relevant treatment goals in psoriasis. Arch Dermatol Res. 2016;308(2):69-78. doi:10.1007/s00403-015-1613-8
- Ryan C, Puig Z, Zema C, et al. Incremental benefits on patient-reported outcomes for achieving PASI 90 or PASI 100 over PASI 75 in patients with moderate to severe psoriasis. Poster presented at: 2018 European Academy of Dermatology and Venerology (EADV) Congress; September 12–16, 2018; Paris, France. Poster 2002.
- Gordon KB, Strober B, Lebwohl M, et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double blind, randomised, placebo controlled and ustekinumab controlled phase 3 trials. Lancet. 2018;392(10148):650-661. doi:10.1016/S0140-6736(18):31713-6
- Puig L. PASI90 response: the new standard in therapeutic efficacy for psoriasis. J Eur Acad Dermatol Venereol. 2015;29(4):645-648. doi:10.1111/jdv.12817
- Strober B, Papp KA, Lebwohl M, et al. Clinical meaningfulness of complete skin clearance in psoriasis. J Am Acad Dermatol. 2016;75(1):77-82.e7. doi:10.1016/j.jaad.2016.03.026
- Gooderham MJ, Papp KA, Lynde CW. Shifting the focus – the primary role of IL-23 in psoriasis and other inflammatory disorders. J Eur Acad Derm Venereol. 2018;32(7):1111-1119. doi:10.1111/jdv.14868
- Gaffen SL, Jain R, Garg AV, Cua DJ. The IL-23-IL-17 immune axis: from mechanisms to therapeutic testing. Nature Rev Immunol. 2014;14(9):585-600. doi:10.1038/nri3707
- Girolomoni G, Strohal R, Puig L, et al. The role of IL-23 and the IL-23/TH17 immune axis in the pathogenesis and treatment of psoriasis. J Eur Acad Dermatol Venereol. 2017;31(10):1616-1626. doi:10.1111/jdv.14433
- Lynde CW, Poulin Y, Vender R, Bourcier M, Khalil S. Interleukin 17A: toward a new understanding of psoriasis pathogenesis. J Am Acad Dermatol. 2014;71(1):141-150. doi:10.1016/j.jaad.2013.12.036
- Warren RB, Blauvelt A, Poulin Y, et al. Efficacy and safety of risankizumab vs. secukinumab in patients with moderate-to-severe plaque psoriasis (IMMerge): results from a phase III, randomized, open-label, efficacy-assessor-blinded clinical trial. Br J Dermatol. 2021;184(1):50-59. doi:10.1111/bjd.19341
- Risankizumab versus secukinumab for subjects with moderate to severe plaque psoriasis. ClinicalTrials.gov identifier: NCT03478787. Updated January 6, 2023. Accessed June 7, 2023. https://clinicaltrials.gov/ct2/show/NCT03478787
- Reich K, Gooderham M, Thaçi D, et al. Risankizumab compared with adalimumab in patients with moderate-to-severe plaque psoriasis (IMMvent): a randomised, double-blind, active-comparator-controlled phase 3 trial. Lancet. 2019;394(10198):576-586. doi:10.1016/S0140-6736(19)30952-3
- Leonardi C, Gordon K, Longcore M, Gu Y, Puig L. Weight-based analysis of psoriasis area and severity index improvement at 52 weeks of risankizumab or ustekinumab treatment: an integrated analysis of patients with moderate-to-severe plaque psoriasis. Poster presented at: 24th World Congress of Dermatology (WCD); June 10–15, 2019; Milan, Italy. Poster 5248.
- Strober B, Menter A, Leonardi C, et al. Efficacy of risankizumab in patients with moderate-to-severe plaque psoriasis by baseline demographics, disease characteristics and prior biologic therapy: an integrated analysis of the phase III UltIMMa-1 and UltIMMa-2 studies. J Eur Acad Dermatol Venereol. 2020;34(12):2830-2838. doi:10.1111/jdv.16521
- Gordon KB, Blauvelt A, Bachelez H, et al. Long-term safety of risankizumab in patients with psoriatic disease: integrated analysis of psoriasis and psoriatic arthritis clinical trial data. Poster presented at: 2023 European Academy of Dermatology and Venereology (EADV) Congress; October 11-13, 2023. Berlin, Germany.
- Gordon KB, Blauvelt A, Coates L, et al. Risankizumab long-term safety in patients with psoriatic disease: integrated analyses of data from psoriasis and psoriatic arthritis clinical trials. Poster presented at: 2022 European Academy of Dermatology and Venereology (EADV) Virtual Congress; September 7-10, 2022. Poster 1607.
- HUMIRA [Summary of Product Characteristics]. AbbVie Deutschland GmbH & Co. KG; October 2022.