SKYRIZI (risankizumab) is an IL-23/p19 inhibitor indicated for the treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic therapy. SKYRIZI, alone or in combination with MTX, is also indicated for the treatment of active psoriatic arthritis in adults who have had an inadequate response or who have been intolerant to one or more DMARDs.1
SIMPLICITY
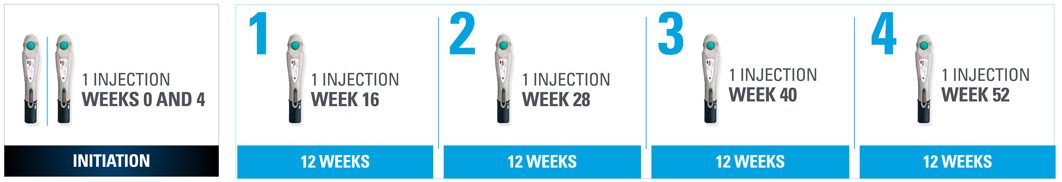
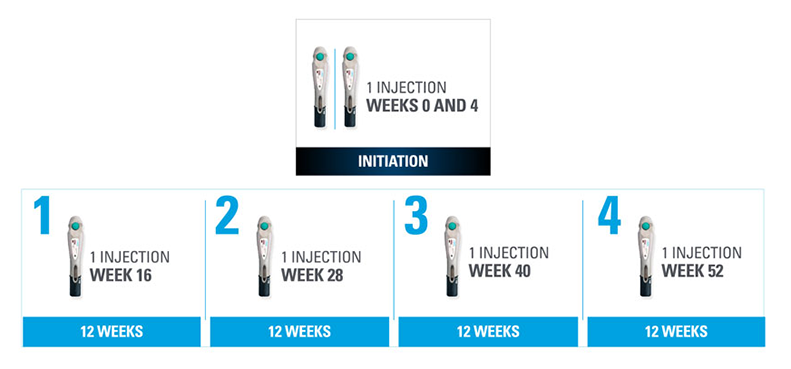
Nothing more than 4 INJECTIONS PER YEAR after initiation doses for both PsO and PsA patients1*
- NO DOSE ADJUSTMENT required regardless of baseline characteristics, including BMI and weight1-3†
- SKYRIZI is dosed 150 mg (one 150-mg subcutaneous injection) at Week 0, Week 4, and every 12 weeks thereafter1
- 1 injection/dose with the freedom to choose either the easy-to-use the SKYRIZI prefilled pen or the prefilled syringe4
Consideration should be given to discontinuing treatment in patients who have shown no response after 16 weeks of treatment. Some plaque psoriasis patients with initial partial response may subsequently improve with continued treatment beyond 16 weeks.
| * | Maintenance dosing (1 injection/dose) every 12 weeks following a starter dose at Week 0 and Week 4. |
| † | Risankizumab clearance and volume of distribution increase as body weight increases, which may result in reduced efficacy in subjects with high body weight (>130 kg). However, this observation is based on a limited number of subjects. |
SKYRIZI one injection per dose:
SAME EFFICACY AND SAFETY PROFILE
Same active ingredient | Demonstrated bioequivalence
NOW EVEN SIMPLER WITH
Note to Affiliate: “New” is a regulated term to be used for up to one year per EFPIA code of practice. Please evaluate use of "Now" in relation to 150 mg presentation, given dates of availability in your country and local requirements.
SKYRIZI 150 mg bioequivalence data1
Bioequivalence was demonstrated between a single SKYRIZI 150-mg injection and two SKYRIZI 75-mg injections in a prefilled syringe. Bioeqivalence was also demonstrated between SKYRIZI 150 mg in a prefilled syringe and a prefilled pen.
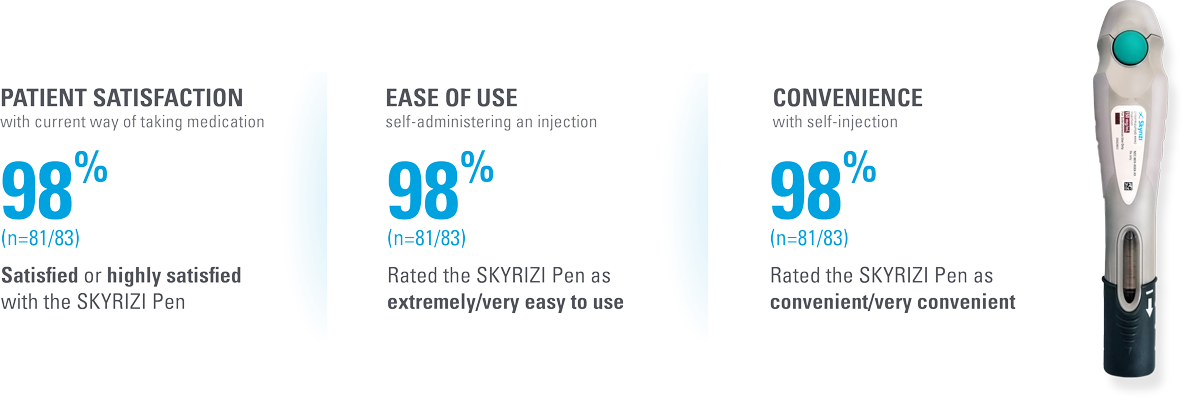
The SKYRIZI prefilled pen scores high across multiple measures of patient satisfaction4*†
| * | Patient satisfaction with self-injection was measured at Week 28 (4 injections total) in patients using the SKYRIZI Pen by the Self-Injection Assessment Questionnaire (SIAQ), a validated instrument for measuring patient feelings and experiences with self-injection. Score measures proportion of subjects achieving the best two categories in post-dose SIAQ by visit (observed cases; intention-to-treat population).4 |
| † | Patient satisfaction was also assessed for (n=83, observed cases): • Satisfied with frequency of injection (97.6%) • Satisfied with duration of injection (96.4%) • Continue self-injecting your medication (100.0%) • Continue self-injecting at home (97.6%) |
ACR=American College of Rheumatology; ADA=adalimumab; AE=adverse events; bio-IR=inadequate response to a biologic; BMI=body mass index; CI=confidence interval; csDMARD-IR=inadequate response to a conventional synthetic DMARD; DLQI=Dermatology Life Quality Index; DMARD=disease-modifying antirheumatic drug; E=event; IBD=inflammatory bowel disease; IL=interleukin; LDI=Leeds Dactylitis Index; LEI=Leeds Enthesitis Index; MACE=major adverse cardiovascular event; MTX=methotrexate; NMSC=nonmelanoma skin cancer; NRI=nonresponder imputation; OC=observed case; OLE=open-label extension; PASI=Psoriasis Area and Severity Index; PBO=placebo; PsA=psoriatic arthritis; PsO=psoriasis; PSS=psoriasis symptom scale; PY=patient-year; RCT=randomized controlled trial; sPGA=static Physician’s Global Assessment; TB=tuberculosis; TEAE=treatment-emergent adverse event; TNF=tumor necrosis factor; UST=ustekinumab.
EU Indications and Important Safety Information for SKYRIZI
INDICATIONS1
Skyrizi (risankizumab) is indicated for the treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic therapy.
Skyrizi, alone or in combination with methotrexate (MTX), is indicated for the treatment of active psoriatic arthritisin adults who have had an inadequate response or who have been intolerant to one or more disease-modifying antirheumaticdrugs (DMARDs).
Skyrizi is indicated for the treatment of adult patients with moderately to severely active Crohn’s disease who have had an inadequate response to, lost response to, or were intolerant to conventional therapy or a biologic therapy.
IMPORTANT SAFETY INFORMATION1
Risankizumab is contraindicated in patients hypersensitive to the active substance or to any of the excipients, and in patients with clinically important active infections (e.g. active tuberculosis). Risankizumab may increase the risk of infection. In patients with a chronic infection, a history of recurrent infection, or known risk factors for infection, risankizumab should be used with caution. Treatment with risankizumab should not be initiated in patients with any clinically important active infection until the infection resolves or is adequately treated.
Patients treated with risankizumab should be instructed to seek medical advice if signs or symptoms of clinically important chronic or acute infection occur. If a patient develops such an infection or is not responding to standard therapy for the infection, the patient should be closely monitored and risankizumab should not be administered until the infection resolves.
Prior to initiating treatment with risankizumab, patients should be evaluated for tuberculosis (TB) infection. Patients receiving risankizumab should be monitored for signs and symptoms of active TB. Anti-TB therapy should be considered prior to initiating risankizumab in patients with a past history of latent or active TB in whom an adequate course of treatment cannot be confirmed.
Prior to initiating therapy with risankizumab, completion of all appropriate immunisations should be considered according to current immunisation guidelines. If a patient has received live vaccination (viral or bacterial), it is recommended to wait at least 4 weeks prior to starting treatment with risankizumab. Patients treated with risankizumab should not receive live vaccines during treatment and for at least 21 weeks after treatment.
If a serious hypersensitivity reaction occurs, administration of risankizumab should be discontinued immediately and appropriate therapy initiated.
The most frequently reported adverse reactions were upper respiratory infections (from 13% in psoriasis to 15.6% in Crohn’s disease). Commonly (≥ 1/100 to < 1/10) reported adverse reactions included tinea infections, headache, pruritus, rash, fatigue, and injection site reactions.
This is not a complete summary of all safety information. Please see the SmPC for complete prescribing information.
Find out more about SKYRIZI
References
- SKYRIZI [Summary of Product Characteristics]. AbbVie Ltd; March 2024.
- Leonardi C, Gordon K, Longcore M, Gu Y, Puig L. Weight-based analysis of psoriasis area and severity index improvement at 52 weeks of risankizumab or ustekinumab treatment: an integrated analysis of patients with moderate-to-severe plaque psoriasis. Presented at: 24th World Congress of Dermatology (WCD); June 10–15, 2019; Milan, Italy. Poster 5248.
- Strober B, Menter A, Leonardi C, et al. Efficacy of risankizumab in patients with moderate-to-severe plaque psoriasis by baseline demographics, disease characteristics and prior biologic therapy: an integrated analysis of the phase III UltIMMa-1 and UltIMMa-2 studies. J Eur Acad Dermatol Venereol. 2020;34(12):2830-2838. doi:10.1111/jdv.16521
- Blauvelt A, Gordon KB, Lee P, et al. Efficacy, safety, usability, and acceptability of risankizumab 150 mg formulation administered by prefilled syringe or by an autoinjector for moderate to severe plaque psoriasis. J Dermatolog Treat. 2022:33(4):2085-2093. doi:10.1080/09546634.2021.1914812