LUMIGAN® (bimatoprost ophthalmic solution)
LUMIGAN® 0.03% MD is indicated for the reduction of elevated intraocular pressure in chronic open-angle glaucoma and ocular hypertension (as monotherapy or as adjunctive therapy to beta-blockers):1
LUMIGAN® (bimatoprost ophthalmic solution 0.01%)
LUMIGAN® (bimatoprost ophthalmic solution 0.03% UD)
What is the efficacy of LUMIGAN® 0.03% MD?
LUMIGAN® 0.03% MD can offer effective IOP-lowering with once-daily dosing1,2
In two studies where patients with glaucoma or OHT treated with 0.03% bimatoprost once daily (QD) (n=474), 0.03% bimatoprost twice daily (BID) (n=483), or 0.5% timolol maleate BID (n=241), once-daily LUMIGAN® 0.03% MD provided significantly lower mean IOP than timolol at every time of the day.3
Overall diurnal mean IOP in patients with glaucoma or OHT who were treated with LUMIGAN® 0.03% MD or timolol maleate for
12 months3

Adapted from Higginbotham E et al. 2002.3
*p<0.001 vs. timolol.
Two identical, multicenter, randomized, double-masked, 1-year clinical trials where patients with glaucoma or OHT were treated with LUMIGAN® 0.03% MD QD (n=474), 0.03% bimatoprost BID (n=483), or 0.5% timolol maleate BID (n=241).3
BID, twice daily; IOP, intraocular pressure; MD, multi dose; OHT, ocular hypertension; QD, once daily.
Mean IOP during the day at baseline and month 12 in patients with glaucoma or OHT who were treated with LUMIGAN® 0.01% or LUMIGAN® 0.03% MD for 12 months2
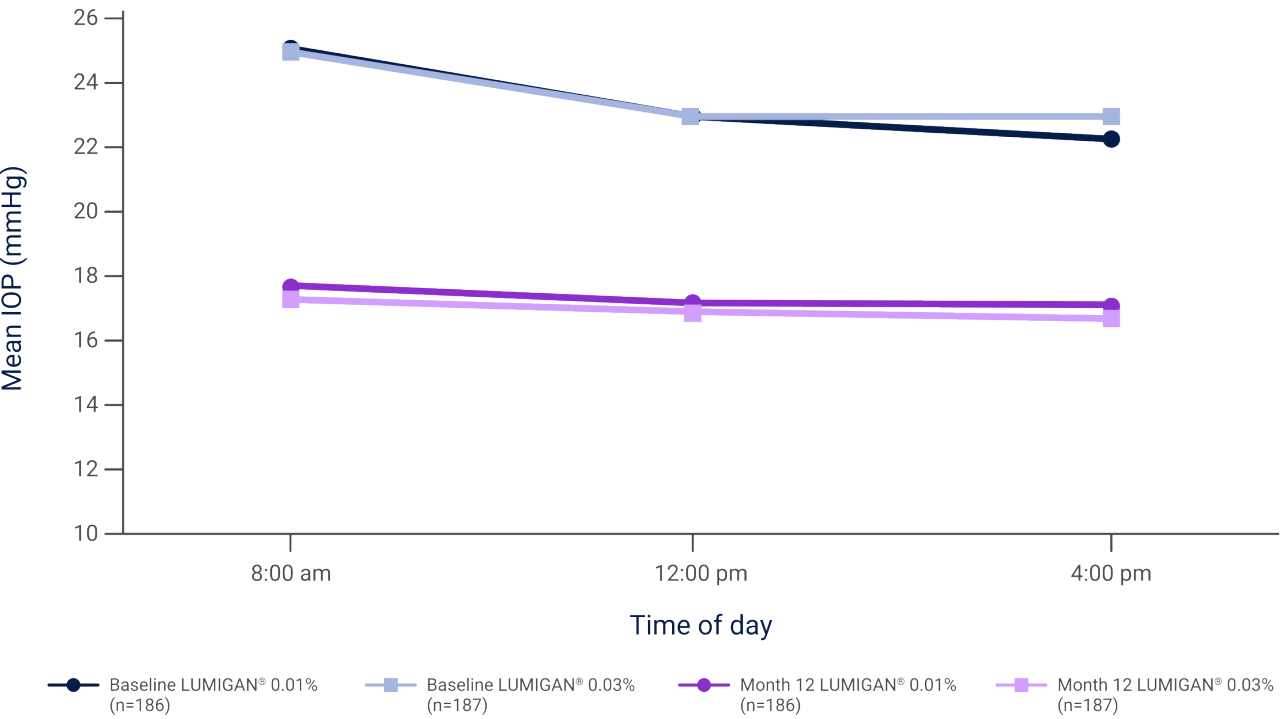
Adapted from Katz L et al. 2010.2
Results of a prospective, randomized, double-masked, multi-center clinical trial evaluating the IOP-lowering efficacy and safety of LUMIGAN® 0.01% (n=186) and LUMIGAN® 0.03% MD (n=187) in patients with glaucoma or ocular hypertension.2
IOP, intraocular reduction; MD, multi dose; OHT, ocular hypertension.
LUMIGAN® 0.03% MD also demonstrated a statistically significant superior reduction in mean morning IOP vs. latanoprost:*,1
• At all visits throughout the 6-month study period, LUMIGAN® 0.03% MD morning mean IOP lowering ranged from -7.6 to -8.2 mmHg vs. -6.0 to -7.2 mmHg with latanoprost
*Multicenter, randomized, investigator-masked clinical trial comparing the IOP-lowering efficacy and safety of topical LUMIGAN® 0.03% MD with latanoprost 0.005%. After washout of glaucoma medications, patients with OHT or glaucoma were randomly assigned to either LUMIGAN® 0.03% MD (n=133) or latanoprost 0.005% (n=136) for 6 months.4
LUMIGAN® 0.03 mg/mL is indicated for the reduction of elevated intraocular pressure in chronic open-angle glaucoma and ocular hypertension in adults (as monotherapy or as adjunctive therapy to beta-blockers).1
In clinical studies, over 1800 patients have been treated with LUMIGAN® 0.03 mg/mL eye drops, solution. On combining the data from Phase III monotherapy and adjunctive LUMIGAN® 0.3 mg/mL eye drops, solution usage, the mot frequently reported treatment-related adverse events were: growth of eyelashes in up to 45% in the first year with the incidence of new reports decreasing to 7% at 2 years and 2% at 3 years, conjunctival hyperaemia (mostly trace to mild and though to be of a non-inflammatory nature) in up to 44% in the first year with the incidence of new reports decreasing to 13% at 2 years and 12% at 3 years and ocular pruritus in up to 14% of patients in the first year with in the incidence of new reports decreasing to 3% at 2 years and 0% at 3 years. Less than 9% of patients discontinued due to any adverse event in the first year with the incidence of additional patient discontinuations being 3% at both 2 and 3 years.1
Please refer to LUMIGAN® 0.03% MD Summary of Product Characteristics for further information on adverse events.


