LUMIGAN® (bimatoprost ophthalmic solution)
LUMIGAN® 0.01% is indicated for the reduction of elevated intraocular pressure in chronic open-angle glaucoma and ocular hypertension (as monotherapy or as adjunctive therapy to beta-blockers)1
LUMIGAN® (bimatoprost ophthalmic solution 0.03% MD)
LUMIGAN® (bimatoprost ophthalmic solution 0.03% UD)
What is LUMIGAN® 0.01% and how does it work?
LUMIGAN® 0.01% belongs to a class of drugs known as prostaglandin analogs1
Prostaglandin analogs like LUMIGAN® 0.01% work by increasing aqueous humor outflow through the trabecular meshwork and enhancing uveoscleral outflow.1
LUMIGAN® 0.01% offers a dual mechanism of action1
The dual mechanism of action stimulates aqueous humor outflow via the trabecular meshwork and uveoscleral pathways, which work together to help lower IOP.1
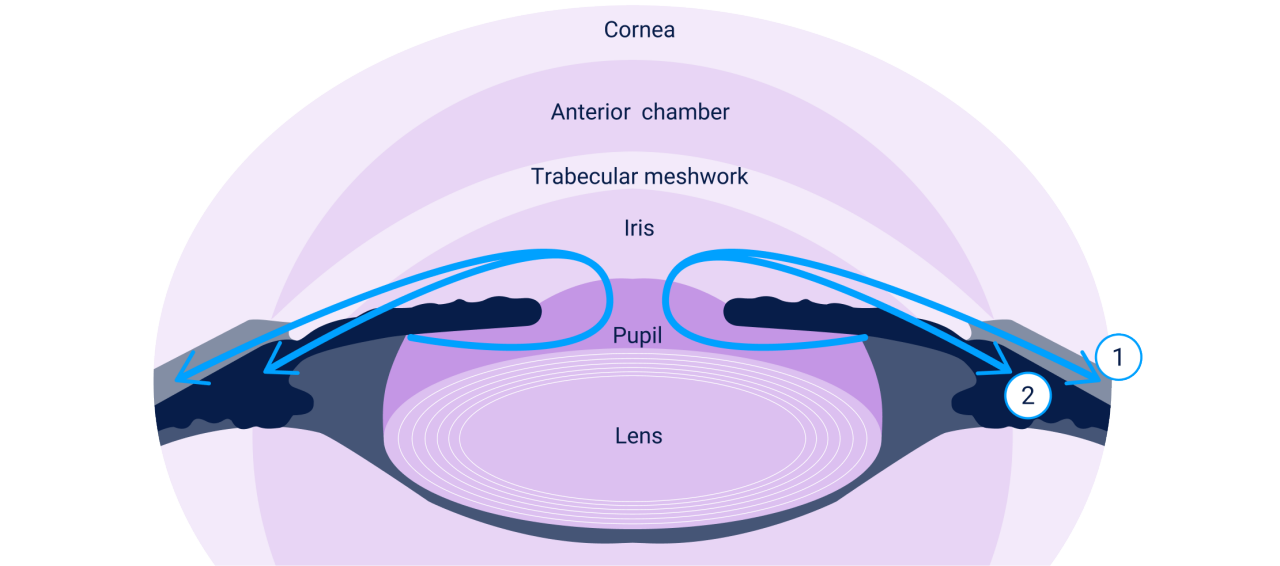
1 – Increases trabecular meshwork outflow1
2 – Increases uveoscleral outflow1
Image adapted from American Academy of Ophthalmology. 2017.2
Copy from LUMIGAN® 0.01% Summary of Product Characteristics. 2022.1
Administration and dosing of LUMIGAN® 0.01%
LUMIGAN® 0.01% offers the convenience of a single once-daily drop.1
LUMIGAN® 0.01% is indicated for the reduction of elevated intraocular pressure in chronic open-angle glaucoma and ocular hypertension in adults (as monotherapy or as adjunctive therapy to beta-blockers)1
LUMIGAN® 0.01% may not be suitable for some of your patients
LUMIGAN® 0.01% is contraindicated in:1
Please refer to LUMIGAN® 0.01% Summary of Product Characteristics for full safety information and precautions for use.
LUMIGAN® 0.01% is indicated for the reduction of elevated intraocular pressure in chronic open-angle glaucoma and ocular hypertension in adults (as monotherapy or as adjunctive therapy to beta-blockers).1
In a 12-month Phase III clinical study approximately 38% of patients treated with LUMIGAN® 0.1 mg/ml eye drops, solution experienced adverse reactions. The most frequently reported adverse reaction was conjunctival hyperaemia (mostly trace to mild and of a non-inflammatory nature) occurring in 29% of patients. Approximately 4% of patients discontinued due to any adverse event in the 12-month study.1
Please refer to LUMIGAN® 0.01% Summary of Product Characteristics for further information on adverse events.



