LUMIGAN® (bimatoprost ophthalmic solution)
LUMIGAN® 0.03% MD is indicated for the reduction of elevated intraocular pressure in chronic open-angle glaucoma and ocular hypertension (as monotherapy or as adjunctive therapy to beta-blockers):1
LUMIGAN® (bimatoprost ophthalmic solution 0.01%)
LUMIGAN® (bimatoprost ophthalmic solution 0.03% UD)
LUMIGAN® 0.03% MD offers you and your patients the reassurance of an established tolerability profile1
The majority of adverse reactions reported in clinical studies or in the post-marketing period using LUMIGAN® 0.03% MD were ocular, mild to moderate, and none were serious.1
In clinical studies, over 1,800 patients have been treated with LUMIGAN® 0.03% MD. The most frequently reported treatment-related adverse events during the first year were:*,1
• Growth of eyelashes (up to 45%)
• Conjunctival hyperemia (mostly trace to mild and thought to be of a non-inflammatory nature) (up to 44%)
• Ocular pruritus (up to 14%)
* Results of a combined analysis of Phase III clinical trials of LUMIGAN® 0.03% MD as monotherapy and adjunctive therapy over 3 years.1
Please refer to LUMIGAN® 0.03% MD Summary of Product Characteristics for further information on safety adverse events.
Very common and common adverse events associated with LUMIGAN® 0.03% in clinical trials or in the post-marketing period1
| System Organ class | Frequency | Adverse reaction |
|---|---|---|
| Nervous system disorders | Common | Headache |
| Eye disorders | Very Common | Conjunctival hyperemia, ocular pruritus, growth of eyelashes, prostaglandin analogue periorbitopathy |
| Common | Superficial punctuate keratitis, corneal erosion, ocular burning, ocular irritation, allergic conjunctivitis, blepharitis, worsening of visual acuity, asthenopia, conjunctival edema, foreign body sensation, ocular dryness, eye pain, photophobia, tearing, eye discharge, visual disturbance/blurred vision, increased iris pigmentation, eyelash darkening, eyelid erythema, eyelid pruritus | |
| Vascular disorders | Common | Hypertension |
| Skin and subcutaneous tissue disorders | Common | Pigmentation of periocular skin |
| Investigations | Common | Liver function test abnormal |
Adapted from LUMIGAN® 0.03% MD. Summary of Product Characteristics. 2022.1
Less than 9% of patients discontinued due to any adverse event in the first year of treatment with LUMIGAN® 0.03% MD1
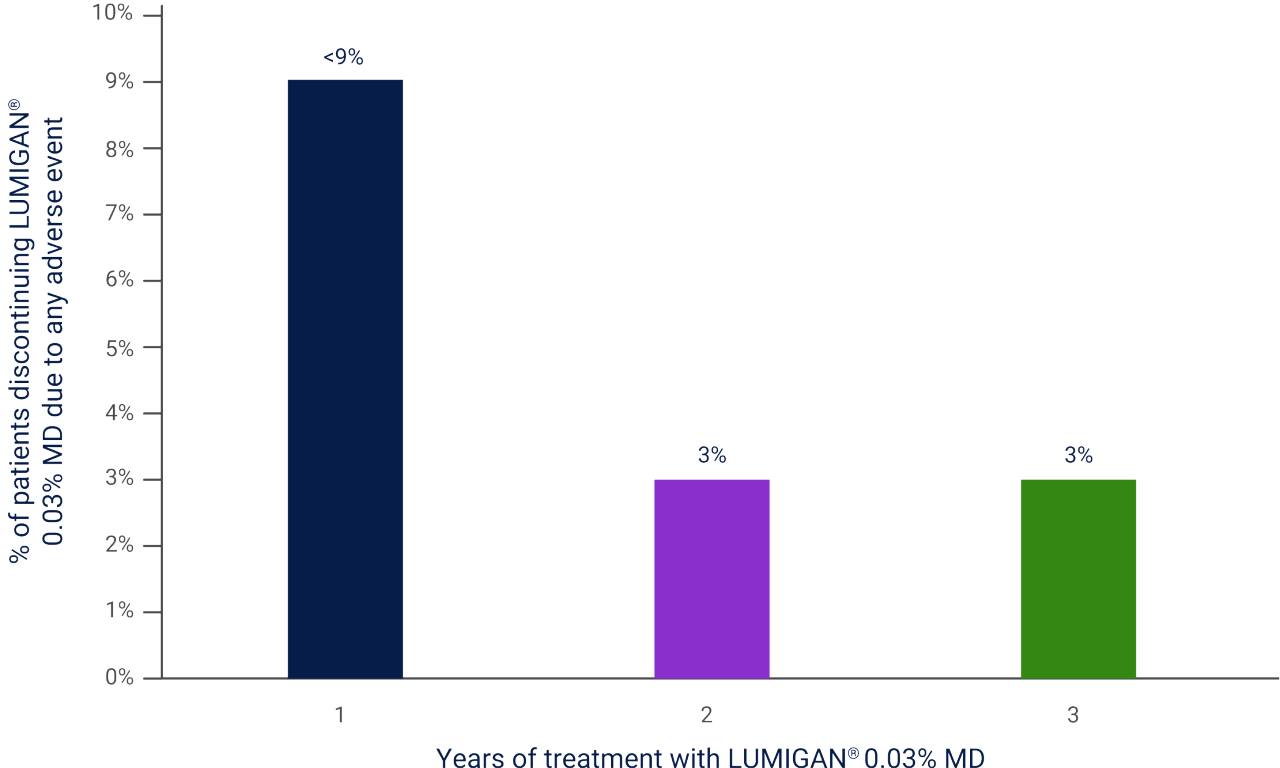
Adapted from LUMIGAN® 0.03% MD Summary of Product Characteristics.1
Results of a combined analysis of Phase III clinical trials of LUMIGAN® 0.03% MD as monotherapy and adjunctive therapy over 3 years.1
MD, multi dose.
After combining data from Phase III monotherapy and adjunctive LUMIGAN® 0.03% MD usage over 3 years:**,1
• Less than 9% of patients discontinued due to any adverse event in the first year
• 3% of patients discontinued after 2 years
• 3% of patients discontinued after 3 years
**Results of a combined analysis of Phase III clinical trials of LUMIGAN® 0.03% MD as monotherapy and adjunctive therapy over 3 years.1
Please refer to the Summary of Product Characteristics for full details of adverse events associated with LUMIGAN® 0.03% MD.
Reporting of suspected adverse reactions
[Placeholder for local markets to populate]
LUMIGAN® 0.03 mg/mL is indicated for the reduction of elevated intraocular pressure in chronic open-angle glaucoma and ocular hypertension in adults (as monotherapy or as adjunctive therapy to beta-blockers).1
In clinical studies, over 1800 patients have been treated with LUMIGAN® 0.03 mg/mL eye drops, solution. On combining the data from Phase III monotherapy and adjunctive LUMIGAN® 0.3 mg/mL eye drops, solution usage, the mot frequently reported treatment-related adverse events were: growth of eyelashes in up to 45% in the first year with the incidence of new reports decreasing to 7% at 2 years and 2% at 3 years, conjunctival hyperemia (mostly trace to mild and though to be of a non-inflammatory nature) in up to 44% in the first year with the incidence of new reports decreasing to 13% at 2 years and 12% at 3 years and ocular pruritus in up to 14% of patients in the first year with in the incidence of new reports decreasing to 3% at 2 years and 0% at 3 years. Less than 9% of patients discontinued due to any adverse event in the first year with the incidence of additional patient discontinuations being 3% at both 2 and 3 years.1
Please refer to LUMIGAN® 0.03% MD Summary of Product Characteristics for further information on adverse events.


