LUMIGAN® (bimatoprost ophthalmic solution)
LUMIGAN® 0.01% is indicated for the reduction of elevated intraocular pressure in chronic open-angle glaucoma and ocular hypertension (as monotherapy or as adjunctive therapy to beta-blockers)1
LUMIGAN® (bimatoprost ophthalmic solution 0.03% MD)
LUMIGAN® (bimatoprost ophthalmic solution 0.03% UD)
What is the efficacy of LUMIGAN® 0.01%?
LUMIGAN® 0.01% can offer effective IOP-lowering with once-daily dosing1–4
During a 12-month pivotal study in adults with glaucoma or OHT who were treated with once-daily LUMIGAN® 0.01%, the mean diurnal IOP values measured at any visit over the 12-month study period differed by no more than 1.1 mmHg throughout the day and were never greater than 17.7 mmHg.1
Mean IOP during the day at baseline and month 12 in patients with glaucoma or OHT who were treated with once-daily LUMIGAN® 0.01% or LUMIGAN® 0.03% MD for 12 months2
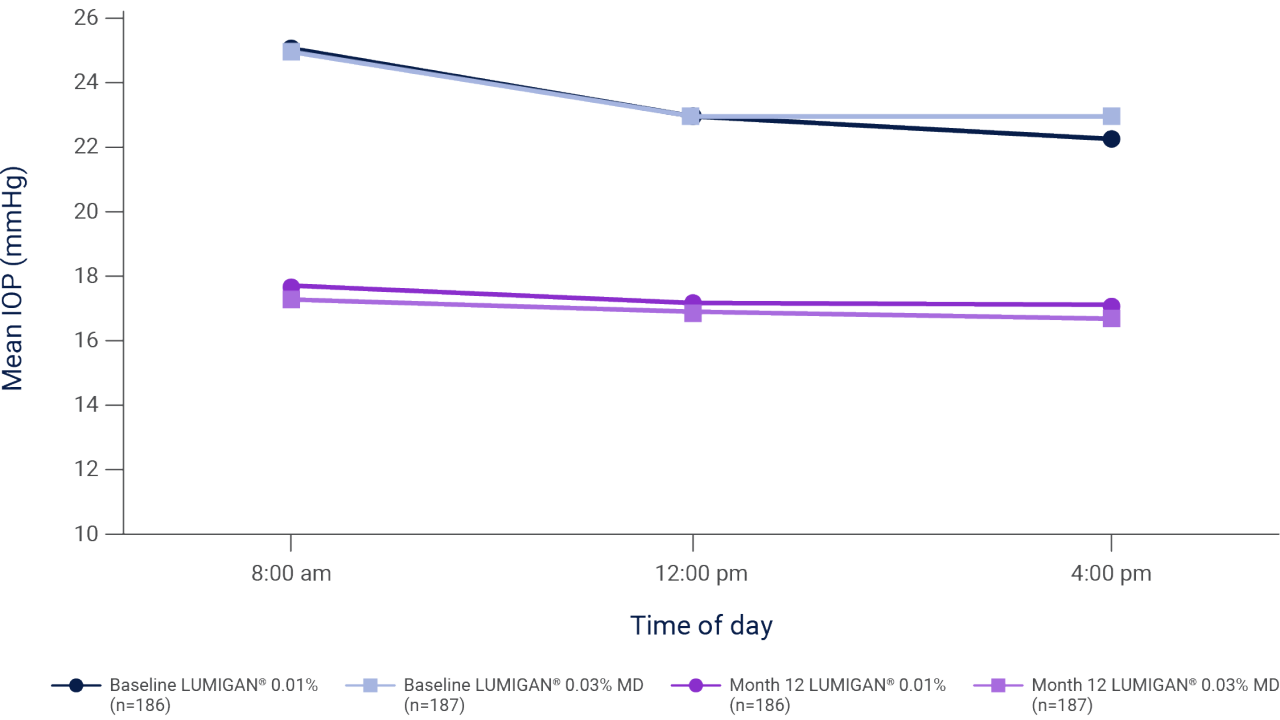
Adapted from Katz L et al. 2010.2
Results of a prospective, randomized, double-masked, multi-center clinical trial evaluating the IOP-lowering efficacy and safety of once-daily LUMIGAN® 0.01% (n=186) and LUMIGAN® 0.03% MD (n=187) in patients with glaucoma or OHT. At month 12, the mean reduction from baseline IOP for LUMIGAN® 0.01% was 7.4 mmHg at 8 am, 5.8 mmHg at 12 pm, and 5.2 mmHg at 4 pm. The baseline mean IOP at 8 am, 12 pm, and 4 pm was 25.1 mmHg, 23 mmHg, and 22.3 mmHg, respectively, in the LUMIGAN® 0.01% group. There were no serious treatment-related adverse events in the study. The most common treatment-related adverse event in both groups was conjunctival hyperemia (28.6% and 26.1% in the LUMIGAN® 0.01% and 0.03% groups, respectively). Only 4 patients (2.2%) in the LUMIGAN® 0.01% group compared with 12 patients (6.4%) in the LUMIGAN® 0.03% MD group discontinued the study early because of ocular adverse events.2
IOP, intraocular reduction; MD, multi dose; OHT, ocular hypertension.
Mean IOP during the day at baseline and month 12 in patients with glaucoma or OHT who were treated with once-daily LUMIGAN® 0.01% for 12 months2
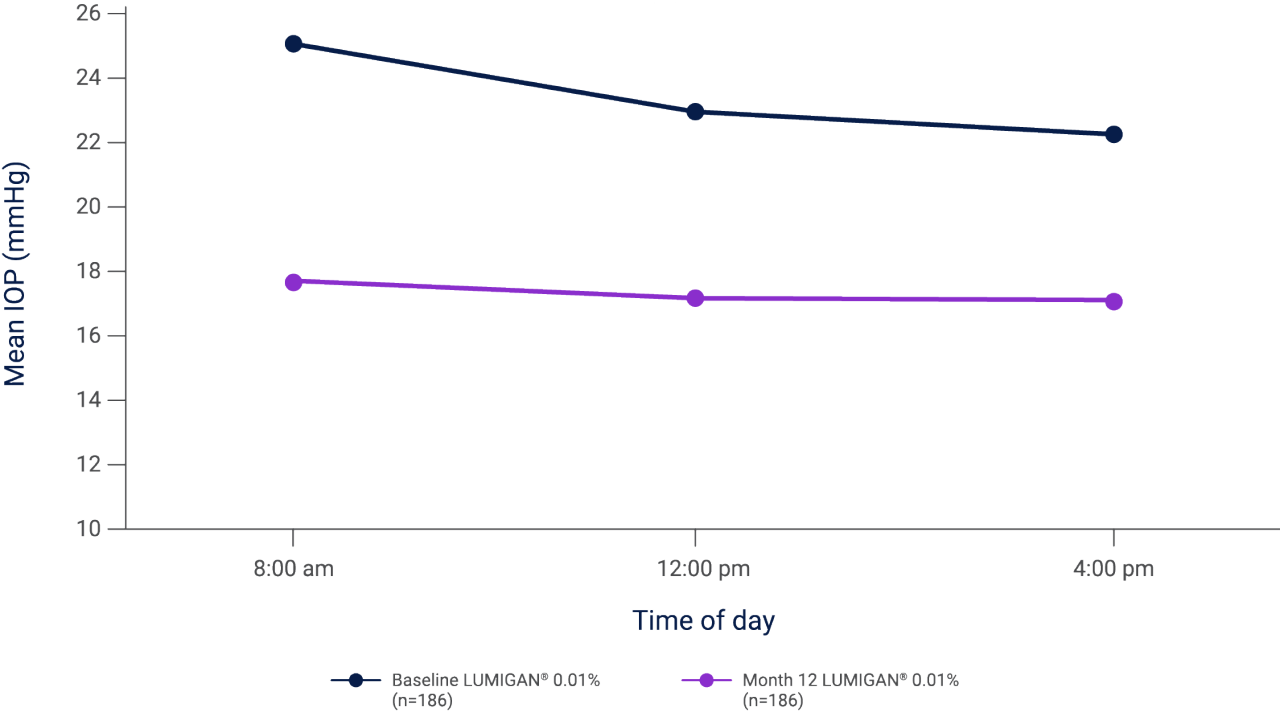
Adapted from Katz L et al. 2010.2
Results of a prospective, randomized, double-masked, multi-center clinical trial evaluating the IOP-lowering efficacy and safety of once-daily LUMIGAN® 0.01% in patients with glaucoma or ocular hypertension.2
IOP, intraocular reduction; OHT, ocular hypertension.
The efficacy of LUMIGAN® 0.01% has also been assessed in
real-world trials3,4
LUMIGAN® 0.01% significantly reduced mean IOP from baseline by -4.1 mmHg (p<0.0001) in patients with POAG or OHT at 10–14 weeks, with the greatest reduction in IOP by 28.5% from baseline (p<0.0001) in treatment-naïve patients (n=1,896).*,3
*Results of a multicenter, prospective, open-label, observational study designed to investigate the efficacy and tolerability of LUMIGAN® 0.01% in routine clinical practice (N=10,337) from 1334 centers in Germany. Baseline IOP was 20.1±4.5 mmHg. AEs were reported in 6.1% of patients (n=629/10,337) with the most commonly reported adverse events being eye irritation (2.0%), ocular hyperemia (1.4%) and conjunctival hyperemia (1.2%).3
Real-world evidence is collected outside of controlled clinical trials and has inherent limitations including a lesser ability to control for confounding factors.
Percentage reduction from baseline in mean IOP in all patients with POAG or OHT and in those receiving prior monotherapy at 10–14 weeks following initiation of LUMIGAN® 0.01%, N=20,136 eyes3
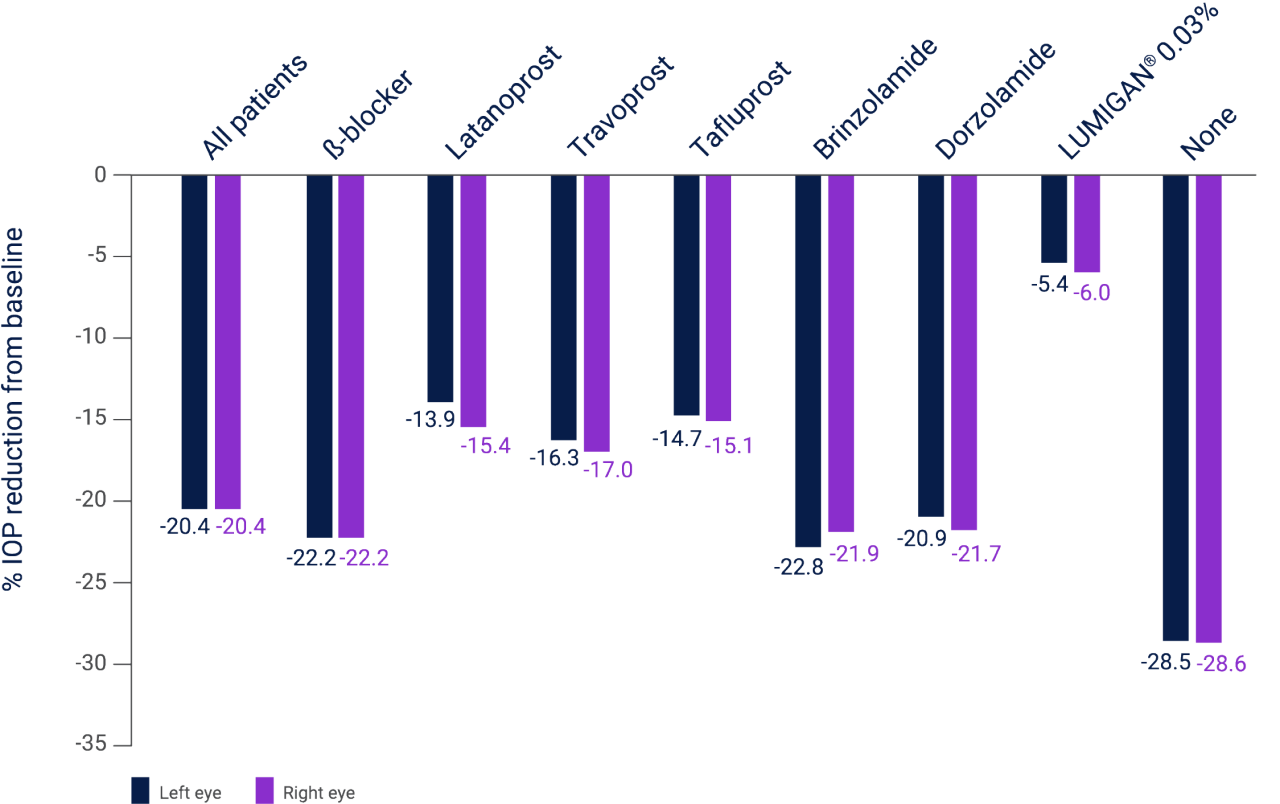
Adapted from Pfennigsdorf S et al. 2012.3
Results of a multicenter, prospective, open-label, observational study investigating the efficacy and tolerability of LUMIGAN® 0.01% in routine clinical practice in patients with and without prior treatment (N=10,337). AEs were reported in 6.1% of patients (n=629/10,337) with the most commonly reported adverse events being eye irritation (2.0%), ocular hyperemia (1.4%) and conjunctival hyperemia (1.2%).3
IOP, intraocular pressure; OHT, ocular hypertension; POAG, primary open-angle glaucoma.
Real-world evidence is collected outside of controlled clinical trials and has inherent limitations including a lesser ability to control for confounding factors.
Mean IOP reduction at 10–14 weeks from baseline in all patients with POAG or OHT receiving LUMIGAN® 0.01% (N=2,593)4
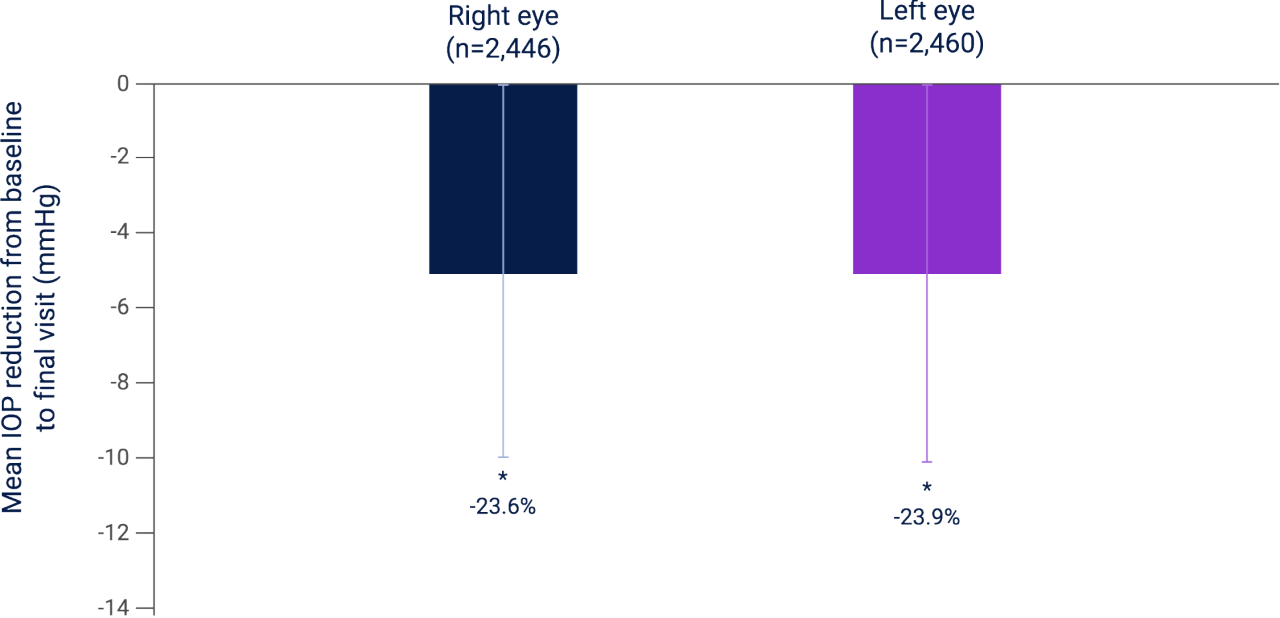
Adapted from Stevens A et al. 2016.4
*p<0.0001.
Results of a combined analysis of four multicenter, prospective, observational studies investigating the IOP-lowering ability, tolerability, and patient adherence to LUMIGAN® 0.01% in patients with POAG or OHT, N=2,593. A total of 328 sites from Austria, Belgium, Switzerland, and the Netherlands were included in this analysis. A total of 78.2% (n=1428/1826) and 77% (n=712/925) of patients with POAG or OHT, respectively, had both eyes included in the analysis. AEs were reported in 16.7% of patients with the most common AEs being conjunctival hyperemia (5.2%) and eye irritation (4.7%).4
IOP, intraocular pressure; OHT, ocular hypertension; POAG, primary open-angle glaucoma.
Real-world evidence is collected outside of controlled clinical trials and has inherent limitations including a lesser ability to control for confounding factors.
The greatest mean IOP reduction was in treatment-naïve patients4
Based on a pooled analysis, LUMIGAN® 0.01% achieved an IOP reduction of 28.8% and 28.9% from baseline in right and left eyes, respectively, in treatment-naïve patients with POAG and OHT after 10–14 weeks†,4
• Mean IOP reduced by 6.7 ± 4.7 mmHg from baseline after 10–14 weeks (p<0.0001, n=2,248 eyes)4
†Results of a combined analysis of four multicenter, prospective, observational studies investigating the IOP-lowering ability, tolerability, and patient adherence to LUMIGAN® 0.01% in patients with POAG or OHT, N=2,593. A total of 328 sites from Austria, Belgium, Switzerland, and the Netherlands were included in this analysis. A total of 78.2% (n=1428/1826) and 77% (n=712/925) of patients with POAG or OHT, respectively, had both eyes included in the analysis. AEs were reported in 16.7% of patients with the most common AEs being conjunctival hyperemia (5.2%) and eye irritation (4.7%).4
Real-world evidence is collected outside of controlled clinical trials and has inherent limitations including a lesser ability to control for confounding factors.
Mean IOP reduction at 10–14 weeks from baseline in treatment-naïve patients with POAG or OHT receiving LUMIGAN® 0.01%, n=2,248 eyes4
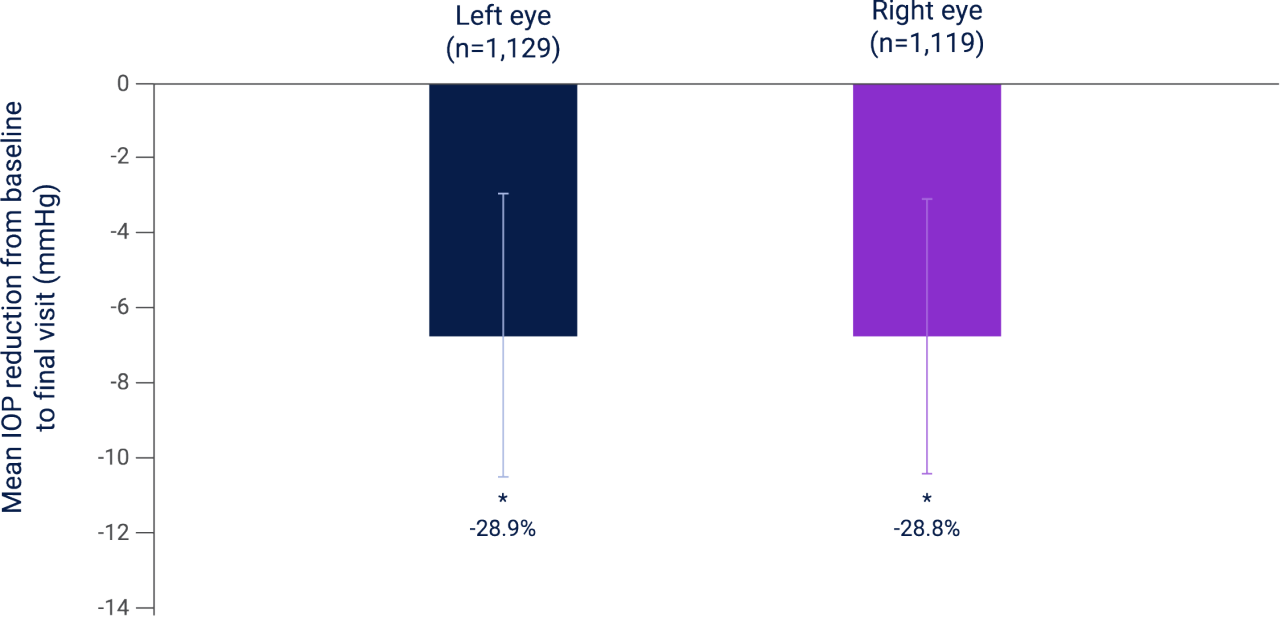
Adapted from Stevens A et al. 2016.4
Results of a combined analysis of four multicenter, prospective, observational studies investigating the IOP-lowering ability, tolerability, and patient adherence to LUMIGAN® 0.01% in patients with POAG or OHT, N=2,593. A total of 328 sites from Austria, Belgium, Switzerland, and the Netherlands were included in this analysis. A total of 78.2% (n=1428/1826) and 77% (n=712/925) of patients with POAG or OHT, respectively, had both eyes included in the analysis. AEs were reported in 16.7% of patients with the most common AEs being conjunctival hyperemia (5.2%) and eye irritation (4.7%).4
IOP, intraocular pressure; OHT, ocular hypertension; POAG, primary open-angle glaucoma.
Real-world evidence is collected outside of controlled clinical trials and has inherent limitations including a lesser ability to control for confounding factors.
LUMIGAN® 0.01% is indicated for the reduction of elevated intraocular pressure in chronic open-angle glaucoma and ocular hypertension in adults (as monotherapy or as adjunctive therapy to beta-blockers).1
In a 12-month Phase III clinical study approximately 38% of patients treated with LUMIGAN® 0.1 mg/ml eye drops, solution experienced adverse reactions. The most frequently reported adverse reaction was conjunctival hyperaemia (mostly trace to mild and of a non-inflammatory nature) occurring in 29% of patients. Approximately 4% of patients discontinued due to any adverse event in the 12-month study.1
Please refer to LUMIGAN® 0.01% Summary of Product Characteristics for further information on adverse events.


