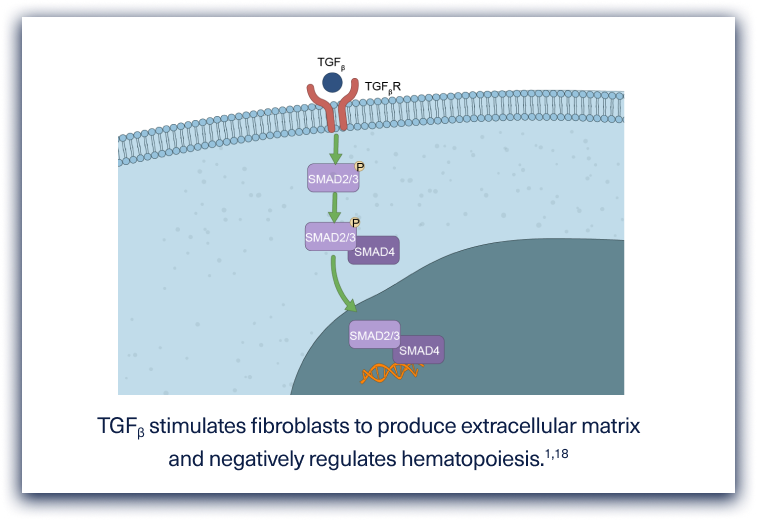
Myelofibrosis is pathologically characterized by deposition of collagen and reticulin fibers and distortion of bony trabeculae.1,5
Patient Molecular Level
Click the pathway tabs to expand details.
Restoration of normal bone marrow appearance coupled with reduction in MPN symptom burden and/or splenomegaly may indicate modification to the underlying disease process.11
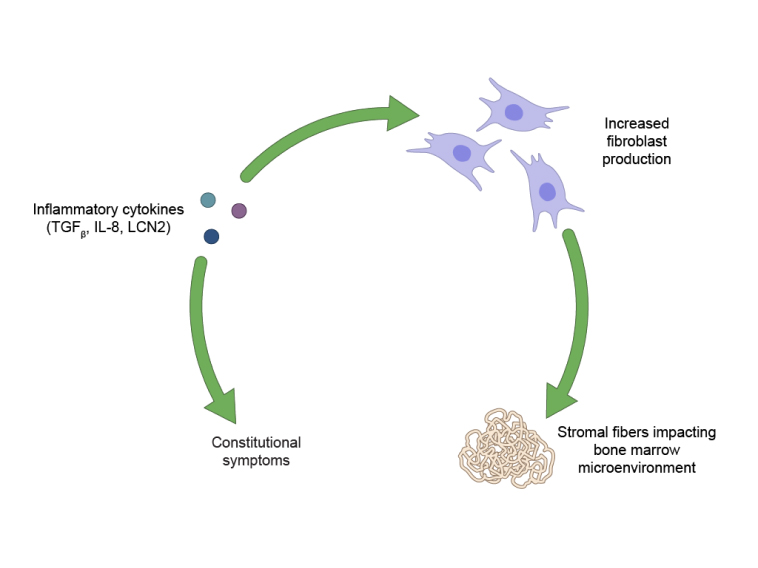
Multiple pathways and key mechanisms leading to disease progression in MF should be further explored.2,12
1. Agarwal A, et al. Bone marrow fibrosis in primary myelofibrosis. Stem Cell Investig. 2016;3:5.
2. Gerds AT. Beyond JAK-STAT: novel therapeutic targets in Ph-negative MPN. Hematology. 2019;2019(1):407-414.
3. Hajmirza A, et al. BET family protein BRD4: an emerging actor in NFkB signaling in inflammation and cancer. Biomedicines. 2018;6(1):16.
4. Jiang Q, Jamieson C. BET’ing on dual JAK/BET inhibition as a therapeutic strategy for myeloproliferative neoplasms. Cancer Cell. 2018;33(1):3-5.
5. Lu M, et al. Lipocalin produced by myelofibrosis cells affects the fate of both hematopoietic and marrow microenvironmental cells. Blood. 2015;126(8):972-982.
6. Melo-Cardenas J, et al. The role of megakaryocytes in myelofibrosis. Hematol Oncol Clin North Am. 2021;35(2):191-203.
7. Mesa RA. The burden of fatigue and quality of life in myeloproliferative disorders (MPDs): an international Internet-based survey of 1179 MPD patients. Cancer. 2007;109(1):68-76.
8. Mitra D, et al. Symptom burden and splenomegaly in patients with myelofibrosis in the United States: a retrospective medical record review. Cancer Med. 2013;2(6):889-898.
9. Mughal TI, Vaddi K, Sarlis NJ, Verstovsek S. Myelofibrosis-associated complications: pathogenesis, clinical manifestations, and effects on outcomes. Int J Gen Med. 2014;7:89-101.
10. Nasillo V, et al. Inflammatory microenvironment and specific T cells in myeloproliferative neoplasms: immunopathogenesis and novel immunotherapies. Int J Mol Sci. 2021;22:1906.
11. Pemmaraju N, et al. Defining disease modification in myelofibrosis in the era of targeted therapy. Cancer. 2022;128:2420-2432.
12. Pettit K, Odenike O. Novel therapies for myelofibrosis. Curr Hematol Malig Rep. 2017;12(6):611-624.
13. Plati J, et al. Apoptotic cell signaling in cancer progression and therapy. Integr Biol. 2011;3(4):279-296.
14. Reilly JT, et al. Guideline for the diagnosis and management of myelofibrosis. Br J Haematol. 2012;158(4):453-471.
15. Tabarroki A, et al. Molecular genetics of myelofibrosis and its associated disease phenotypes. Transl Med UniSa. 2014;8:53-64.
16. Tremblay D, Mascarenhas J. Next generation therapeutics for the treatment of myelofibrosis. Cells. 2021;10:1034.
17. Wang X, et al. Imetelstat, a telomerase inhibitor is capable of depleting myelofibrosis stem and progenitor cells. Blood Adv. 2018;2(18):2378-2388.
18. Yanagida M, et al. The role of transforming growth factor-β in PEG-ruHuMGDF-induces reversible myelofibrosis in rats. Br J Haematol. 1997;99:739-745.
19. Zahr AA, et al. Bone marrow fibrosis in myelofibrosis: pathogenesis, prognosis and targeted strategies. Haematologica. 2016;101(6):660-671.







 (1).png)


.png)
.png)

.png)
.png)
.png)

.png)
.png)