Improve your patients’ function to help them get back to what matters1–3
AQUIPTA™ met all patient-reported outcome endpoints across two 12-week pivotal studies*1–3
Help improve the function of your patients with AQUIPTA™:
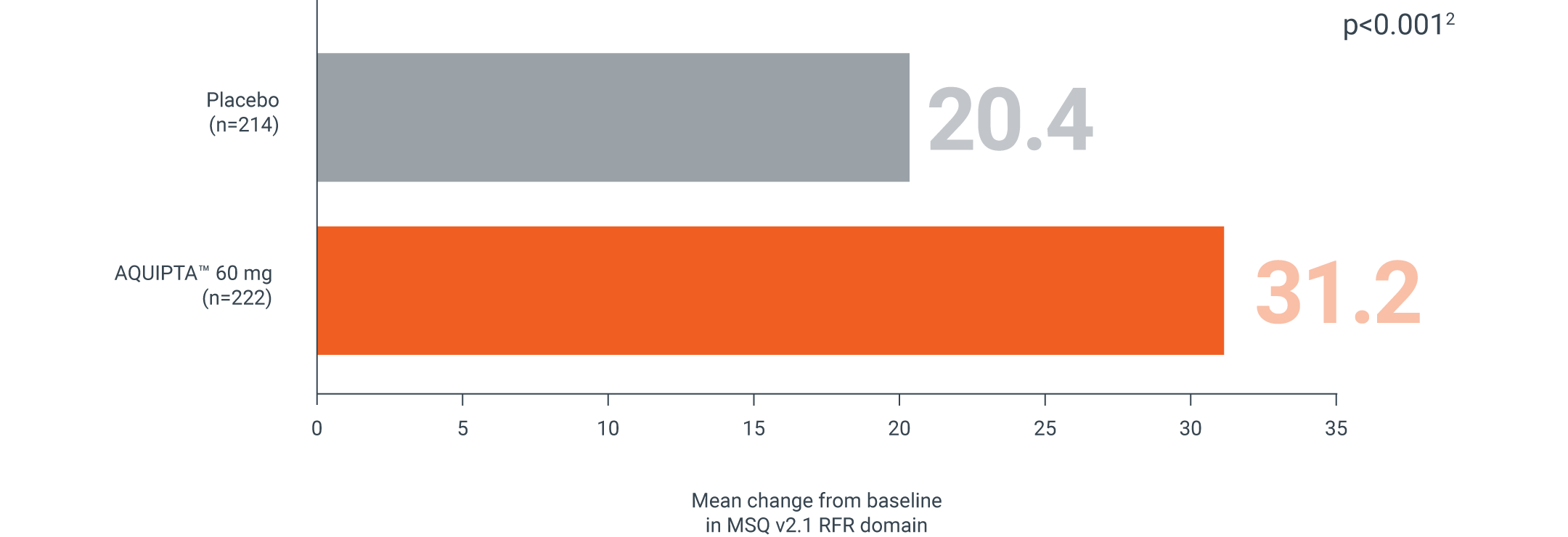
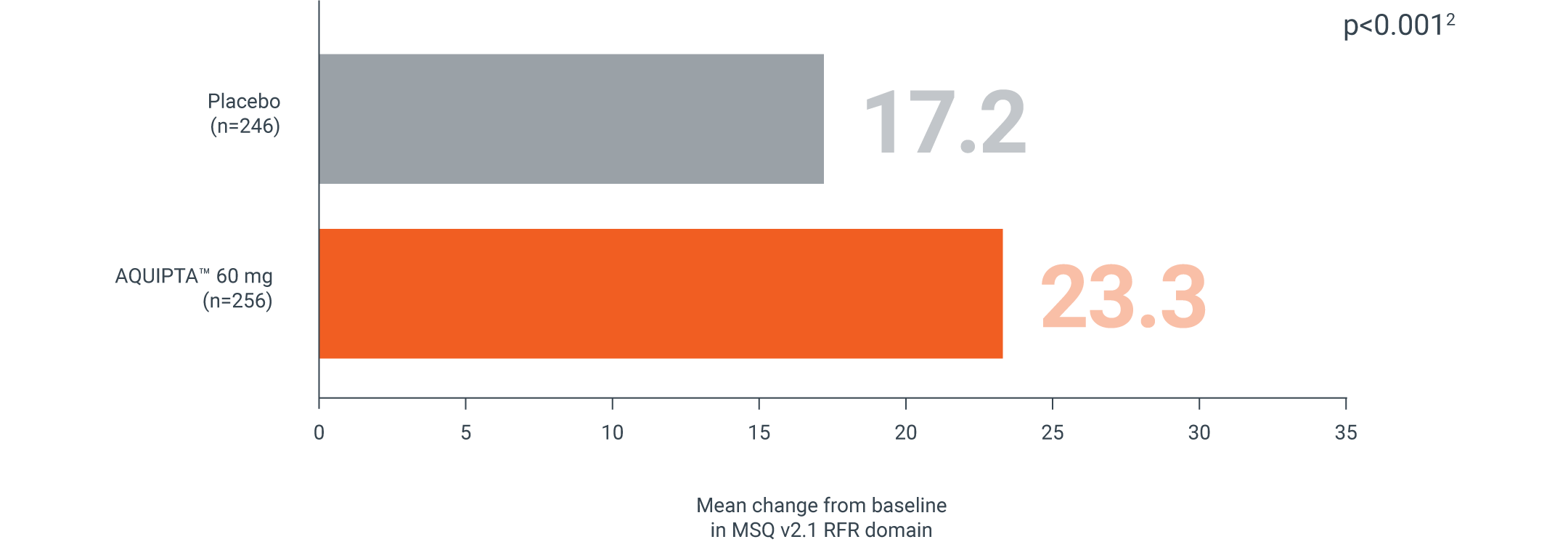
MSQ v2.1 RFR
EPISODIC MIGRAINE, 60 MG | ADVANCE PIVOTAL PREVENTION STUDY†
AQUIPTA™ reduces how often migraine impacts daily social- and work-related activities vs. placebo‡1,2
Mean change from baseline in MSQ v2.1 RFR domain at Week 122
Secondary endpoint:
Adapted from Ailani J, et al.2
mITT population: AQUIPTA™ 60 mg: 46.8 baseline.2 Placebo: 46.8 baseline.2
An increase in score from baseline indicates improvement.2 The minimally important difference (MID) is 3.2.4
CHRONIC MIGRAINE, 60 MG | PROGRESS PIVOTAL PREVENTION STUDY¶
AQUIPTA™ reduces how often migraine impacted daily social- and work-related activities vs. placebo**1,3
Mean change from baseline in MSQ v2.1 RFR domain at Week 123
Secondary endpoint:
Adapted from Pozo-Rosich P, et al.3
mITT population: AQUIPTA™ 60 mg: 43.6 baseline.3 Placebo: 43.6 baseline.3
An increase in score from baseline indicates improvement.3 MID is 3.2.4
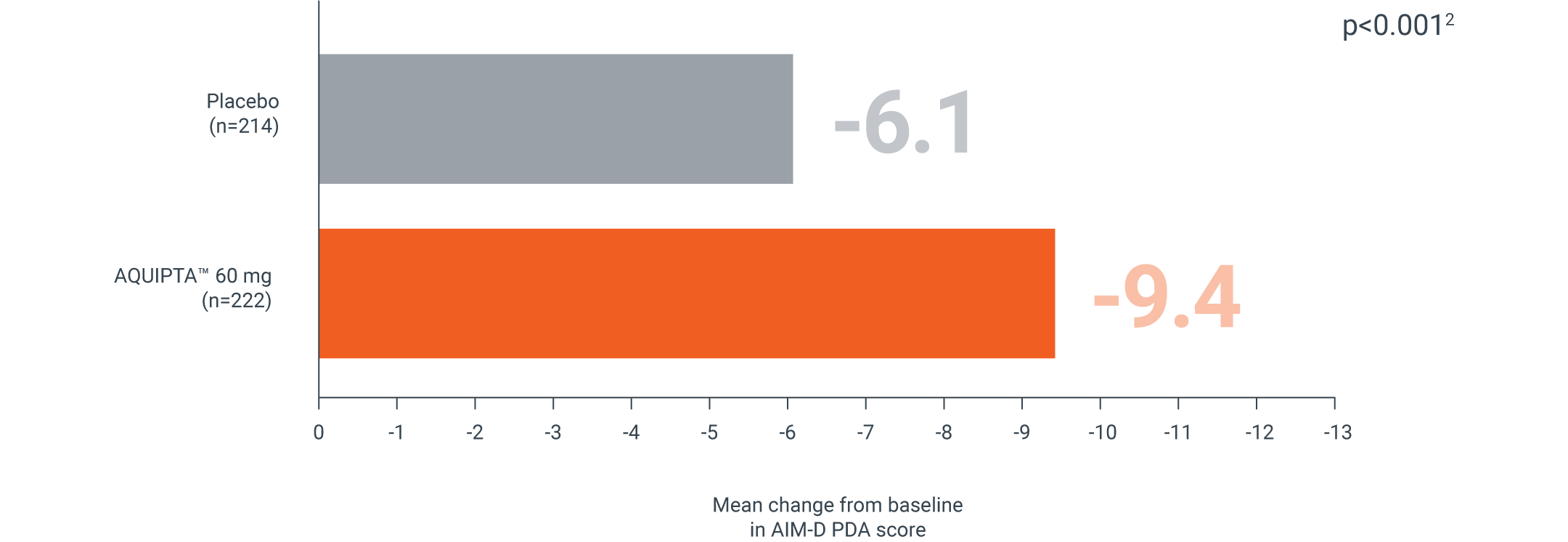
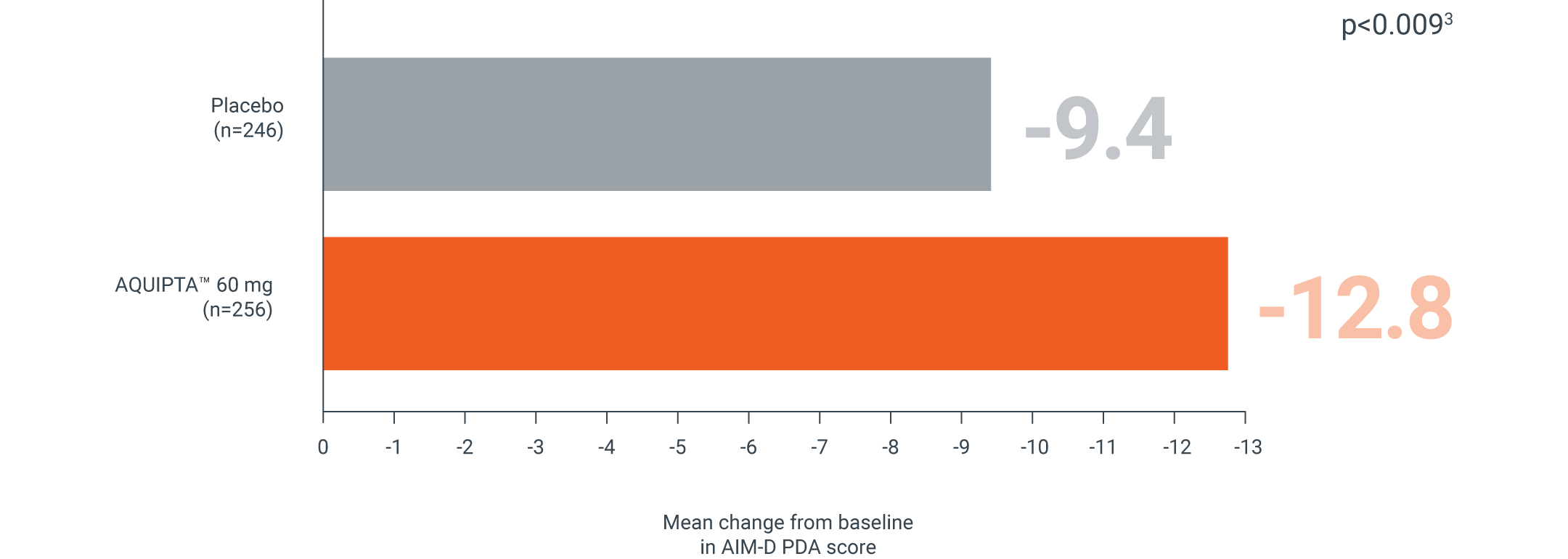
AIM-D
EPISODIC MIGRAINE, 60 MG | ADVANCE PIVOTAL PREVENTION STUDY†
AQUIPTA™ helps improve your patients’ ability to perform daily activities vs. placebo§1,2
Mean change from baseline in AIM-D PDA score across 12 weeks‖2
Secondary endpoint:
Adapted from Ailani J, et al.2
mITT population: AQUIPTA™ 60 mg: 15.9 baseline.2 Placebo: 15.2 baseline.2
Scale measure 0–100. A reduction in scores from baseline indicates improvement.5
CHRONIC MIGRAINE, 60 MG | PROGRESS PIVOTAL PREVENTION STUDY¶
AQUIPTA™ helps improve your patients’ ability to perform daily activities vs. placebo††1,3
Mean change from baseline in AIM-D PDA score across 12 weeks‖3
Secondary endpoint:
Adapted from Pozo-Rosich P, et al.3
mITT population: AQUIPTA™ 60 mg: 31.2 baseline.3 Placebo: 29.5 baseline.3
Scale measure 0–100. A reduction in scores from baseline indicates improvement.5
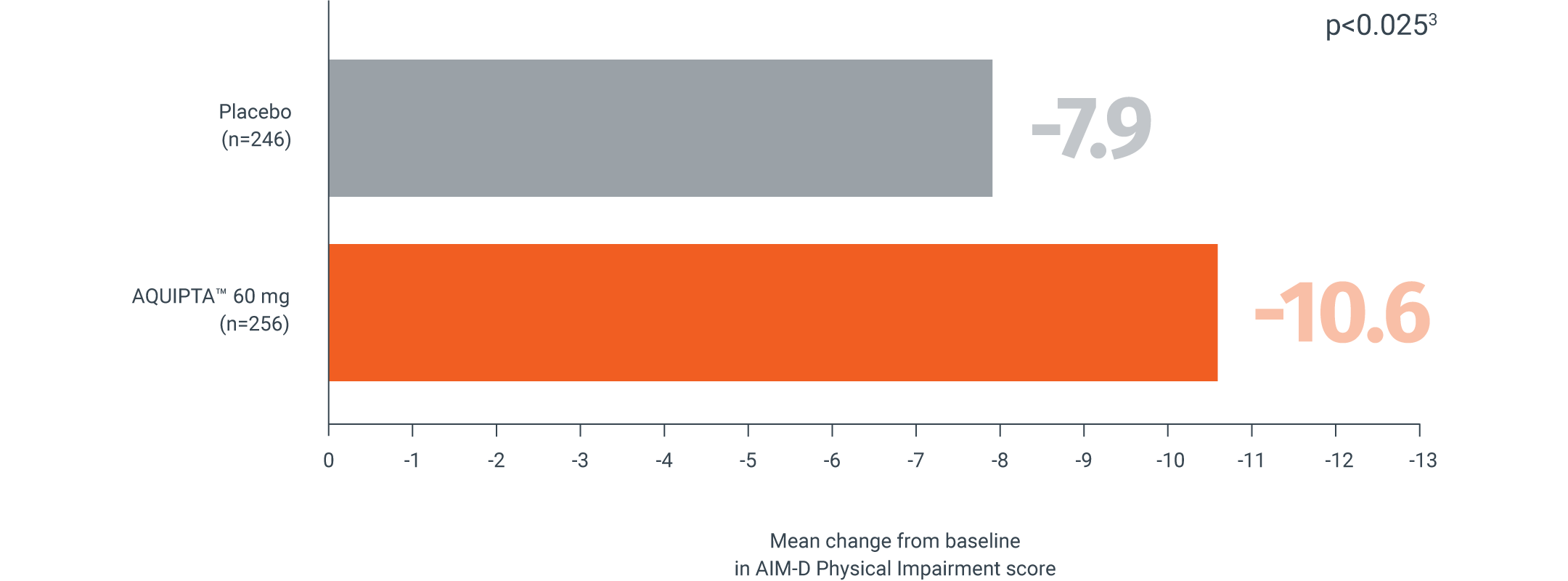
AQUIPTA™ helps improve your patients’ movement vs. placebo††1,3
Mean change from baseline in AIM-D Physical Impairment score across 12 weeks‖3
Secondary endpoint:
Adapted from Pozo-Rosich P, et al.3
mITT population: AQUIPTA™ 60 mg: 27.1 baseline.3 Placebo: 25.2 baseline.3
Scale measure 0–100. A reduction in scores from baseline indicates improvement.5
AIM-D, Activity Impairment in Migraine-Diary; ICHD, International Classification of Headache Disorders; IE, Ireland; MID, minimally important difference; mITT, modified intent-to-treat; MSQ, Migraine-Specific Quality of Life Questionnaire; PDA, Performance of Daily Activities; PI, Physical Impairment; RFR, Role Function-Restrictive.
*Additional endpoints in the episodic migraine and chronic migraine pivotal clinical trials included change from baseline at Week 12 for MSQ v2.1 RFR domain score and the AIM-D PDA and PI scores. The MSQ v2.1 RFR domain score assesses how often migraine impacts function related to daily social and work-related activities. An increase in scores from baseline indicates improvement. The AIM-D evaluates difficulty with performance of daily activities (PDA domain) and physical impairment (PI domain) due to migraine.2,3 Higher scores indicate a greater effect of migraine and a reduction in scores from baseline indicates improvement. †AQUIPTA™ was evaluated for the prophylaxis of episodic migraine. The episodic migraine study (ADVANCE) enrolled patients who met ICHD criteria for a diagnosis of migraine with or without aura.1,2 ‡Additional endpoints in the ADVANCE study included change from baseline at Week 12 for MSQ v2.1 RFR domain score that assesses how often migraine impacts daily social and work-related activities. An increase in scores from baseline indicates improvement. Efficacy analyses used the mITT population that included all participants who received ≥1 dose of AQUIPTA™ or placebo and had evaluable baseline, and ≥1 4-week post-baseline, eDiary data.2 §Additional endpoints in the ADVANCE study included change from baseline at Week 12 for the AIM-D PDA score. The AIM-D evaluates difficulty with performance of daily activities due to migraine. Higher scores indicate a greater effect of migraine and a reduction in scores from baseline indicates improvement. Efficacy analyses used the mITT population that included all participants who received ≥1 dose of AQUIPTA™ or placebo and had evaluable baseline, and ≥1 4-week post-baseline, eDiary data.2 ‖The AIM-D is a content valid and psychometrically sound measure of activity impairment with migraine. The robust quantitative data support evaluation of the PDA domain as an endpoint in clinical trials of preventive treatments in patients with episodic or chronic migraine. Future research is needed to further evaluate meaningful changes for the AIM-D domains.5 ¶AQUIPTA™ was evaluated for the prophylaxis of chronic migraine. The chronic migraine study (PROGRESS) enrolled patients who met ICHD criteria for chronic migraine.1,3 **Additional endpoints in the PROGRESS study included change from baseline at Week 12 for MSQ v2.1 RFR domain score that assesses how often migraine impacts daily social and work-related activities. An increase in scores from baseline indicates improvement.3 ††Change from baseline at Week 12 for the AIM-D PDA score was an additional secondary endpoint in all regions other than Canada and Europe.3 The AIM-D evaluates difficulty with performance of daily activities due to migraine. Higher scores indicate a greater effect of migraine and a reduction in scores from baseline indicates improvement. Efficacy analyses used the mITT population that included all participants who received ≥1 dose of AQUIPTA™ or placebo and had evaluable baseline, and ≥1 4-week post-baseline, eDiary data. ‡‡Free from migraine defined as 0 attacks in a defined period.
References:
- AQUIPTA™ (atogepant) IE Summary of Product Characteristics. August 2023.
- Ailani J, et al. N Engl J Med. 2021;385:695–706.
- Pozo-Rosich P, et al. Lancet. 2023;402(10404):775–785 (Supplementary Appendix).
- Cole JC, et al. Cephalalgia. 2009;29(11):1180–1187.
- Lipton RB, et al. Headache. 2022;62(1):89–105.
ALL-AQP-230003 March 2024