Choose migraine prevention that’s effective in reducing monthly migraine days*1
EPISODIC MIGRAINE, 60 MG | ADVANCE PIVOTAL PREVENTION STUDY*
Primary endpoint:
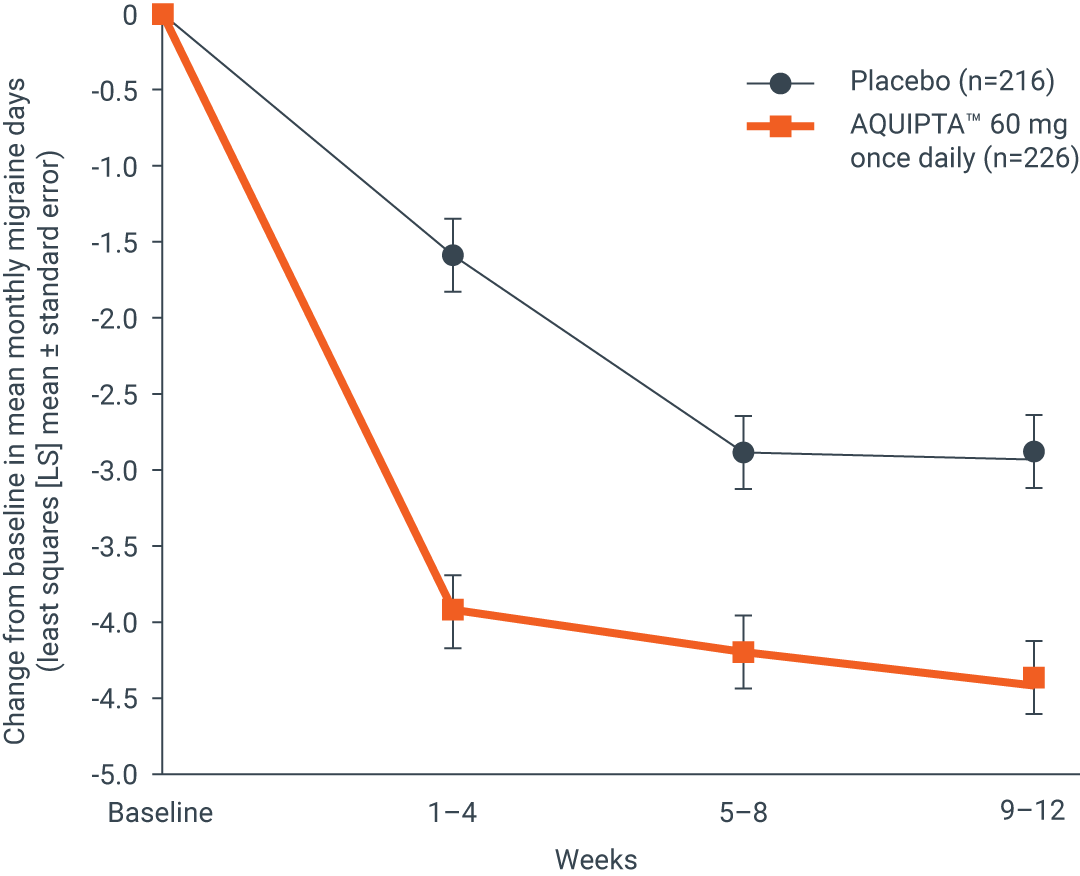
Across 12 weeks, there was a significant reduction in mean monthly migraine days from baseline by1
52.6%
for patients treated with
AQUIPTA™ 60 mg once daily1
vs.
33.3%
for patients treated
with placebo1
p<0.0011
Change from baseline in mean monthly
migraine days across 12 weeks1
Adapted from AQUIPTA™ (atogepant) IE Summary of Product Characteristics. August 2023.1
AQUIPTA™ 60 mg (n=226): 4.1 fewer mean monthly migraine days (from 7.8 baseline).1 Placebo (n=216): 2.5 fewer mean monthly migraine days (from 7.5 baseline).1 Difference from placebo: –1.7.1
Results based on the off-treatment hypothetical estimand (OTHE) population.
From the safety population, the most commonly-reported AEs (≥5%) in ADVANCE were constipation (AQUIPTA™ 60 mg 6.9% vs. placebo 0.5%) and nausea (AQUIPTA™ 60 mg 6.1% vs. placebo 1.8%). AQUIPTA™ 60 mg n=231, placebo n=222.*2
CHRONIC MIGRAINE, 60 MG | PROGRESS PIVOTAL PREVENTION STUDY*
Primary endpoint:
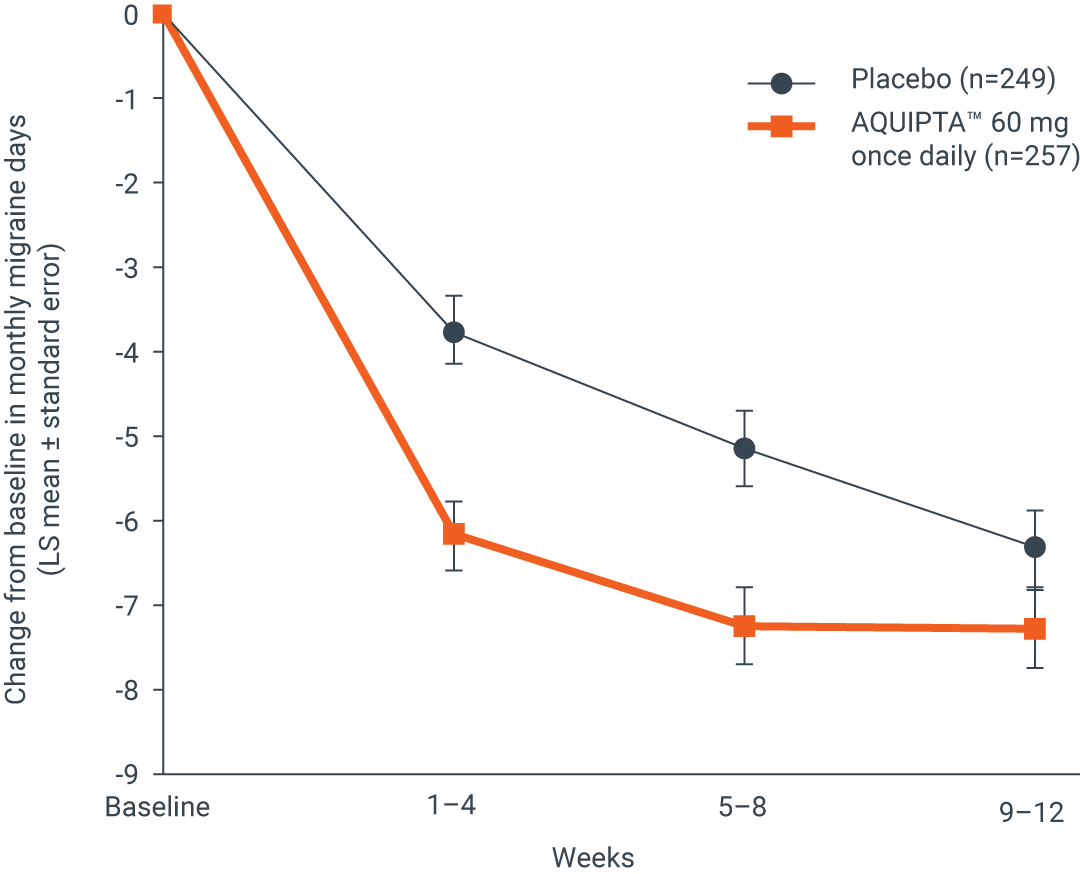
Across 12 weeks, there was a significant reduction in mean monthly migraine days from baseline by1
35.4%
for patients treated with
AQUIPTA™ 60 mg once daily1
vs.
26.8%
for patients treated
with placebo1
p=0.0021
Change from baseline in mean monthly
migraine days across 12 weeks1
Adapted from AQUIPTA™ (atogepant) IE Summary of Product Characteristics. August 2023.1
AQUIPTA™ 60 mg (n=257): 6.8 fewer mean monthly migraine days (from 19.2 baseline).1 Placebo (n=249): 5.1 fewer mean monthly migraine days (from 19.0 baseline).1 Difference vs. placebo: –1.7.1
Results based on the OTHE population.
From the safety population, the most commonly-reported TEAEs (≥5%) in PROGRESS were constipation (AQUIPTA™ 60 mg 10% vs. placebo 3%), nausea (AQUIPTA™ 60 mg 10% vs. placebo 4%) and dizziness (AQUIPTA™ 60 mg 5% vs. placebo 3%). AQUIPTA™ 60 mg n=261, placebo n=255.*3
AQUIPTA™ offers your patients with episodic migraine:
EPISODIC MIGRAINE, 60 MG | ADVANCE PIVOTAL PREVENTION STUDY‡
Exploratory endpoint:
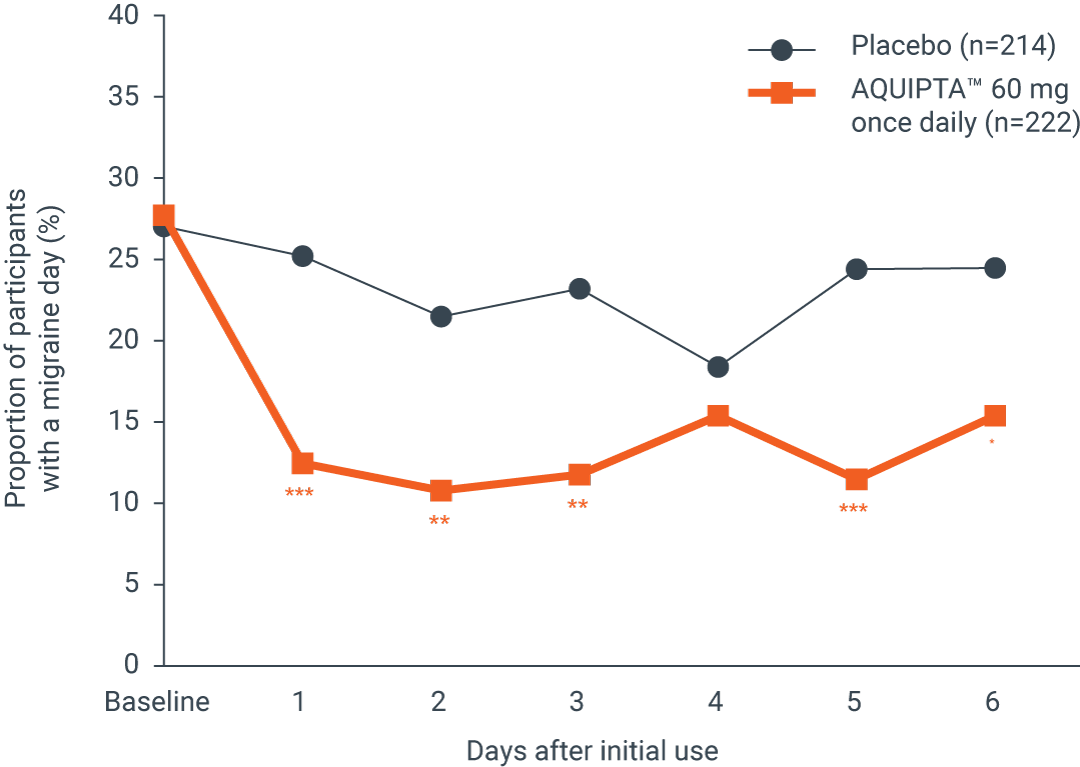
At Week 1, there was a significant reduction in mean weekly migraine days from baseline of4
52.6%
for patients treated with
AQUIPTA™ 60 mg once daily4
vs.
15.8%
for patients treated
with placebo4
p<0.00014
Proportion of patients with a migraine on each
day during the first week of treatment†4
Adapted from Schwedt TJ, et al.4
*p<0.05; **p<0.01; ***p<0.001.
mITT population: AQUIPTA™ 60 mg (n=222): 1.0 fewer weekly migraine days from 1.9 at baseline.4 Placebo (n=214): 0.3 fewer weekly migraine days from 1.9 weekly migraine days at baseline.4
EPISODIC MIGRAINE, 60 MG | 52-WEEK OPEN-LABEL SAFETY STUDY
Efficacy endpoint:
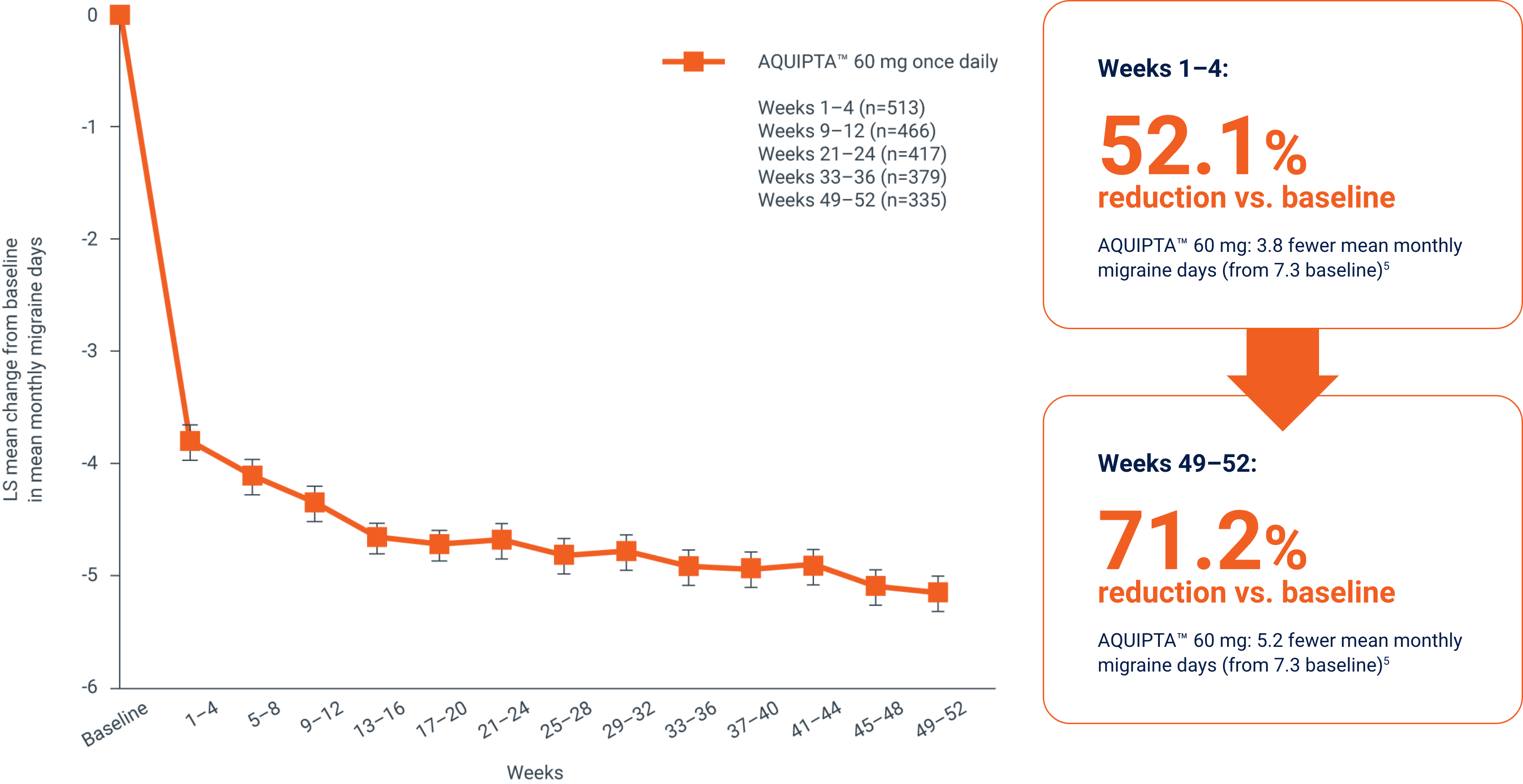
At the end of 1 year, (Weeks 49–52),1,5
reductions in mean monthly migraine days were sustained, with a
71.2%
reduction vs. baseline in those patients
who completed treatment1,5
Change from baseline in mean monthly
migraine days across 1 year5
Adapted from Ashina M, et al.5
Efficacy endpoints for long-term efficacy evaluation were not classified as primary, secondary or additional endpoints. Data from an open-label safety study that randomised 744 patients 5:2 to receive either AQUIPTA™ 60 mg (N=546) or standard of care migraine prevention medication (n=198). Adults from the previous Phase 2b/3 trial, who re-established study eligibility, and de novo patients were included. Participants had 4–14 migraine days in the 28-day baseline period. Efficacy measures, changes from baseline in least squares mean monthly migraine days, moderate/severe headache days, mean monthly acute medication use days and the proportion of responders based on reductions in monthly migraine days were evaluated using the mITT population and a mixed-effects model for repeated measures. Clinical efficacy outcomes were only collected from the AQUIPTA™ arm by eDiary data. Of N=546 randomised to the AQUIPTA™ 60 mg arm, n=521 were included in the mITT population and n=373 completed 52 weeks’ treatment. AQUIPTA™ 60 mg: 5.2 fewer mean monthly migraine days (from 7.3 baseline).5 In an open-label extension with observed data, there is potential for enrichment of the long-term data as those who remain in the study generally fare better than those who discontinue.
AQUIPTA™ offers your patients with chronic migraine:
CHRONIC MIGRAINE, 60 MG | PROGRESS PIVOTAL PREVENTION STUDY*
Secondary endpoint:
Across 12 weeks,1
40%
of patients treated with AQUIPTA™
60 mg once daily1
vs.
27%
of patients treated
with placebo1
achieved a ≥50% reduction in mean
monthly migraine days from baseline1
p=0.0021
AQUIPTA™ 60 mg (n=257). Placebo (n=249). OR (95% CI):
1.9 (1.29 to 2.79).1 Results based on the OTHE population.
AE, adverse event; CI, confidence interval; ICHD, International Classification of Headache Disorders; IE, Ireland; LS, least squares; mITT, modified intent-to-treat;
OR, odds ratio; OTHE, off-treatment hypothetical estimand; TEAE, treatment-emergent adverse event.
*AQUIPTA™ was evaluated for the prophylaxis of migraine in two pivotal studies. The episodic migraine study (ADVANCE) enrolled patients who met ICHD criteria for a diagnosis of migraine with or without aura. The chronic migraine study (PROGRESS) enrolled patients who met ICHD criteria for chronic migraine.1–3 †The proportion of patients with a migraine on any given day during the baseline period ranged from 26.6–28.1% across treatment groups (calculated by dividing the baseline monthly migraine days by 28). On Day 1 after the first AQUIPTA™ dose, 14.1% (10 mg), 10.8% (30 mg) and 12.3% (60 mg) reported a migraine day vs. 25.2% for placebo (p≤0.0071 for all AQUIPTA™ groups). The difference between AQUIPTA™ and placebo did not reach significance on Day 3, Day 4 and Day 6 (10 mg) and Day 4 (60 mg).4 ‡An additional analysis of a pivotal study that evaluated AQUIPTA™ for the prophylaxis of episodic migraine that enrolled patients who met ICHD criteria for a diagnosis of migraine with or without aura.1,2,4 Efficacy analyses used the mITT population that included all participants who received ≥1 dose of AQUIPTA™ or placebo and had evaluable baseline, and ≥1 4-week post-baseline, electronic diary (eDiary) data.4
References:
- AQUIPTA™ (atogepant) IE Summary of Product Characteristics. August 2023.
- Ailani J, et al. N Engl J Med. 2021;385:695–706.
- Pozo-Rosich P, et al. Lancet. 2023;402(10404):775–785 (Supplementary Appendix).
- Schwedt TJ, et al. Cephalalgia. 2022;42(1):3–11.
- Ashina M, et al. Headache. 2023;63(1):79–88.
ALL-AQP-230003 March 2024