DELETE ALL ON PAGE AND DE-ACTIVATE THIS CLINICAL STUDIES LANDING PAGE
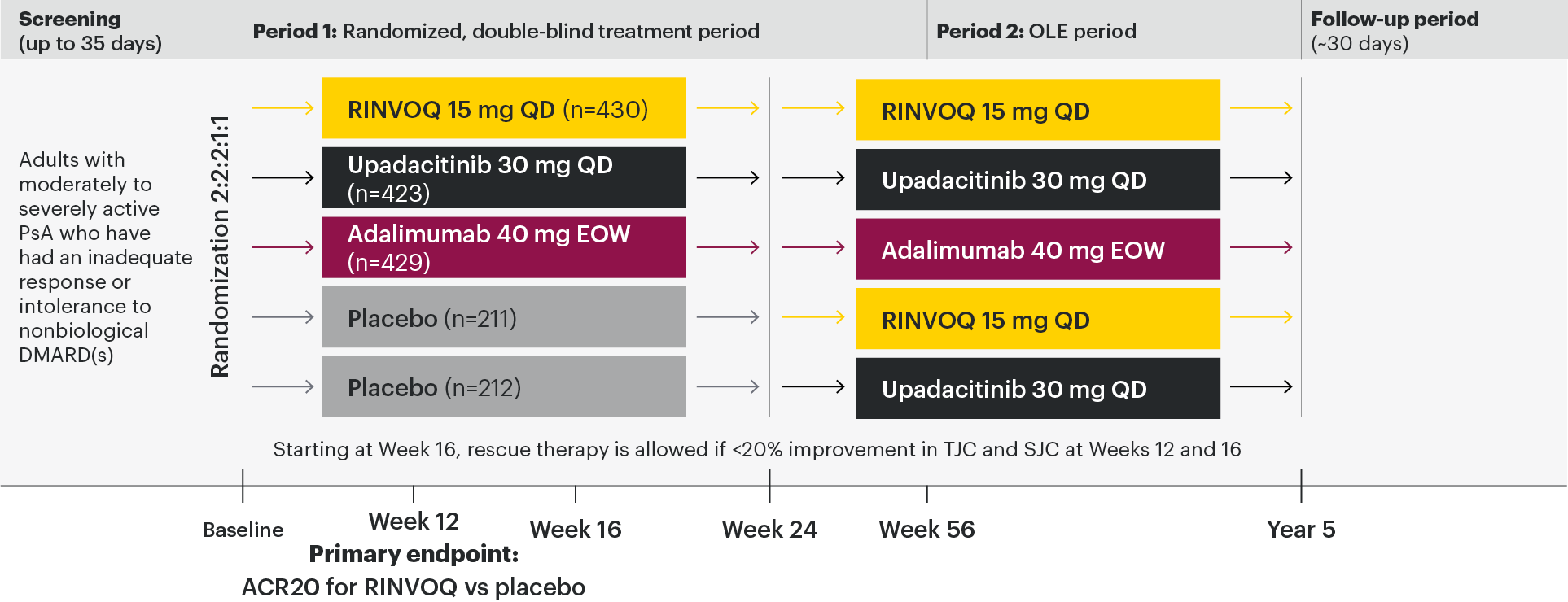
A Phase 3 study investigating the efficacy and safety of RINVOQ compared with placebo and adalimumab for the treatment of active PsA in patients with an inadequate response to nonbiologic DMARDs1,2
The approved dose of RINVOQ is 15 mg once daily. Upadacitinib 30 mg QD is not an approved dose in PsA.
All subjects received x-rays of hands and feet at screening, Week 24, Week 56, Week 104, and Week 152. At Week 16, rescue therapy was offered to subjects classified as nonresponders (defined as not achieving at least 20% improvement in tender joint count and swollen joint count at both Week 12 and Week 16). At Week 24, all placebo subjects were switched to RINVOQ 15 mg QD or upadacitinib 30 mg QD (1:1 ratio) regardless of response.
Primary
ACR20 response for RINVOQ vs placebo at Week 12
Key ranked secondary endpoints
(RINVOQ vs placebo unless noted)
- Change from baseline in HAQ-DI at Week 12
- Proportion of subjects achieving a slGA of Psoriasis of 0 or 1 and at least a 2-point improvement from baseline at Week 16
- PASI 75 response at Week 16
- Change from baseline mTSS at Week 24
- Proportion of subjects achieving MDA at Week 24
- Proportion of subjects with resolution of enthesitis (LEl=0) at Week 24
- ACR20 response rate at Week 12 (noninferiority vs adalimumab)
- Change from baseline in SF-36 PCS at Week 12
- Change from baseline in FACIT-F at Week 12
- ACR20 response rate at Week 12 (superiority vs adalimumab)*
- Proportion of subjects with resolution of dactylitis (LDl=0) at Week 24†
- Change from baseline in patient’s assessment of pain NRS at Week 12 (superiority vs adalimumab)†
- Change from baseline in HAQ-DI at Week 12 (superiority vs adalimumab); and†
- Change from baseline in SAPS at Week 16†
Safety
Data for treatment-emergent adverse events and laboratory assessments were collected during the study. Treatment-emergent adverse events were defined as adverse events that began or worsened in severity after the first dose of study medication through 30 days after the last dose.
*Superiority of RINVOQ 15 mg vs adalimumab could not be demonstrated, which prevented the testing of significance for secondary endpoints lower in the testing hierarchy.
†Not tested for significance / not multiplicity-controlled; no clinical inferences can be drawn from these data.
- ≥18 years old at screening
- Clinical diagnosis of PsA with symptom onset ≥6 months prior to screening and fulfillment of the CASPAR criteria
- Active disease at baseline defined as ≥3 tender joints and ≥3 swollen joints
- Presence at screening of either ≥1 erosion on x-ray as determined by central imaging review or hs-CRP >ULN
- Diagnosis of active plaque psoriasis or documented history of plaque psoriasis
- Inadequate response or intolerance to treatment with at least one nonbiologic DMARD*
- On ≤2 nonbiologic DMARDs
- Prior exposure to any JAK inhibitor
- Prior exposure to any bDMARD
At baseline, 1,393 (82%) of patients were on at least 1 concomitant non-bDMARD; 1,084 (64%) of patients received concomitant MTX, only; and 311 (18%) of patients were on monotherapy.
*Lack of efficacy after ≥12 weeks of therapy; intolerance or contraindication as defined by investigator
ACR20: improvement of at least 20% in the American College of Rheumatology core criteria; bDMARD: biological disease-modifying antirheumatic drug; CASPAR: Classification Criteria for Psoriatic Arthritis; DMARD: disease-modifying antirheumatic drug; EOW: every other week; hs-CRP: high-sensitivity C-reactive protein; JAK: Janus kinase; MTX: methotrexate; OLE: open-label extension; QD: once daily; SJC: swollen joint count; TJC: tender joint count; ULN: upper limit of normal.
In patients with active PsA and an inadequate response to nonbiologic DMARDs
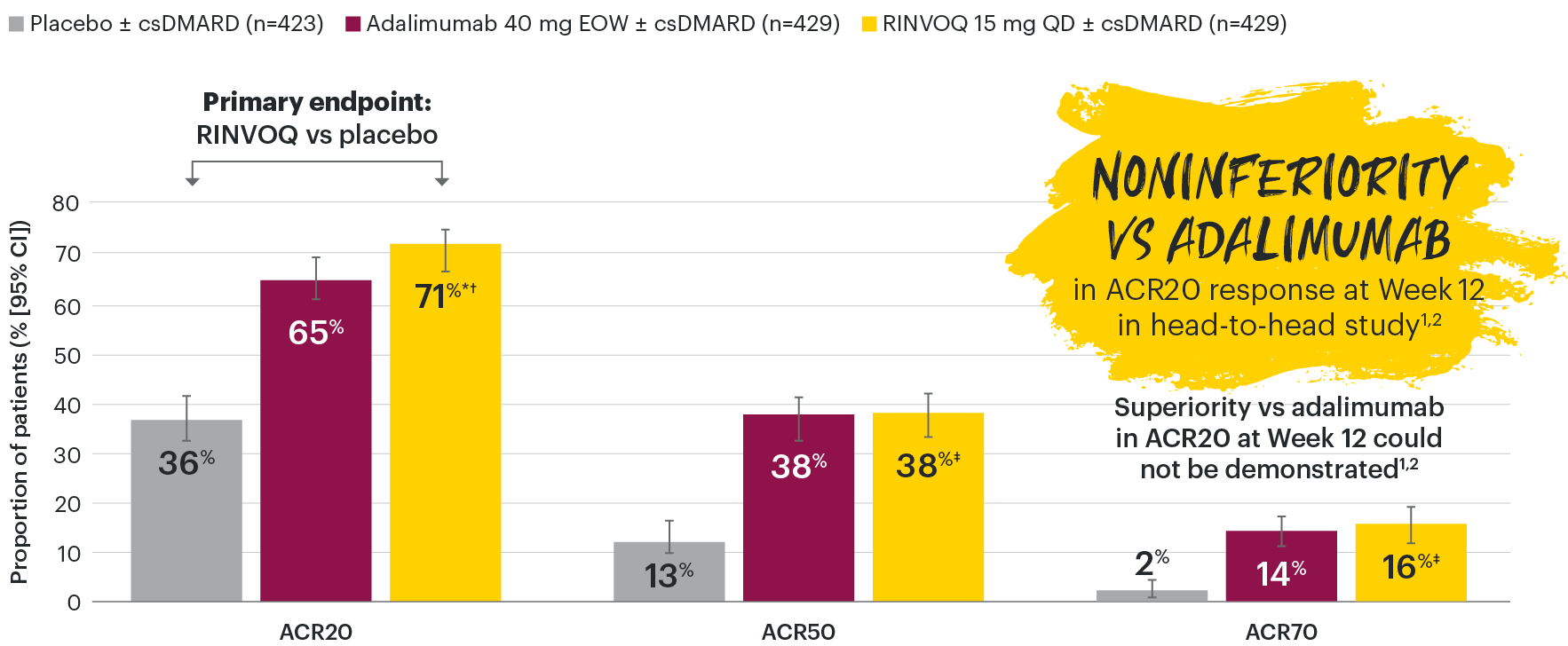
RINVOQ joint symptom improvement was superior to placebo for ACR20 at Week 12
SELECT-PsA 1: ACR20/50/70 response rates at Week 12 (NRI)1-3
NOTE TO AFFILIATES: Please evaluate y-axis scale throughout, per local regulations.
*P≤0.001 vs placebo, statistically significant in the multiplicity-controlled analysis.
†P≤0.001 vs adalimumab for noninferiority, statistically significant in the multiplicity-controlled analysis.
‡Nominal P≤0.001 vs placebo. No clinical inferences can be drawn.3
ACR20 for RINVOQ vs placebo at Week 12 was a primary multiplicity-controlled endpoint.
ACR20 noninferiority vs adalimumab at Week 12 was a key ranked secondary, multiplicity-controlled endpoint.
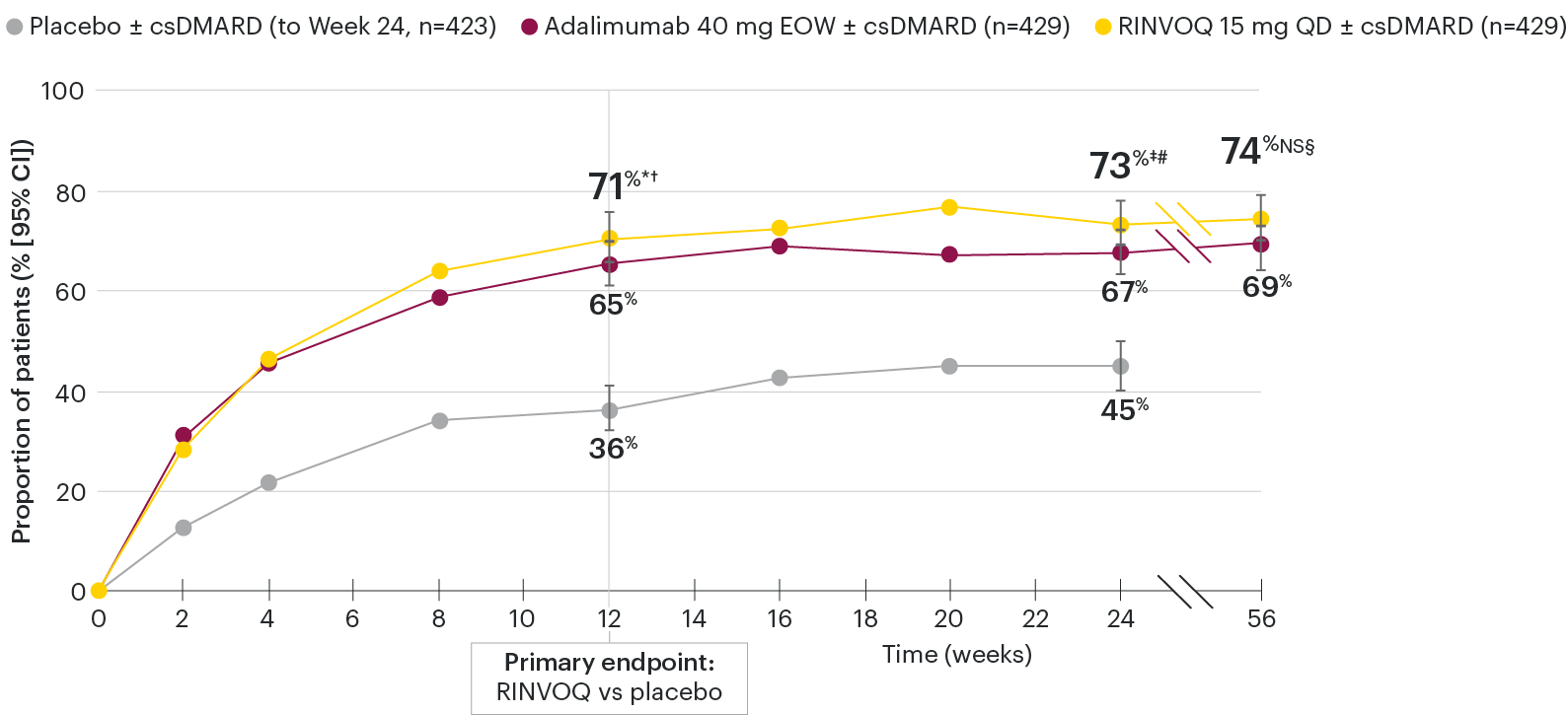
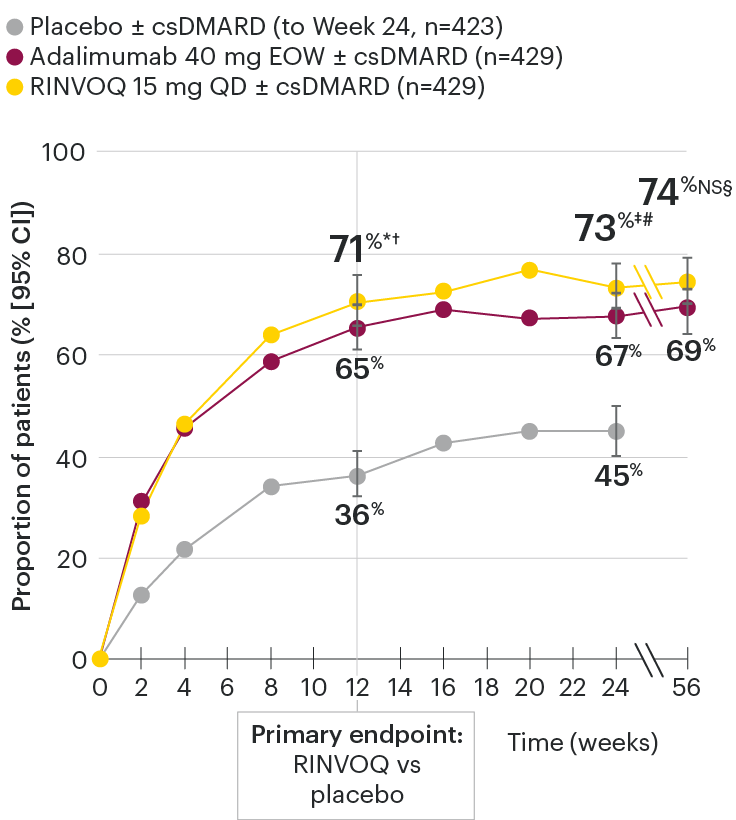
In patients with active PsA and an inadequate response to nonbiologic DMARDs
ACR20 response rates over time
SELECT-PsA 1: ACR20 response rates (NRI)1-4
*P≤0.001 vs placebo, statistically significant in the multiplicity-controlled analysis.
†P≤0.001 vs adalimumab for noninferiority, statistically significant in the multiplicity-controlled analysis.
‡Nominal P≤0.001 vs placebo.3
#Nominal P≤0.05 vs adalimumab.3
§Not significant (nominal P=0.06) vs adalimumab.4
Nominal P-values denote data not controlled for multiplicity. No clinical inferences can be drawn.
Superiority vs adalimumab in ACR20 at Week 12 could not be demonstrated.2
At Week 24, all patients randomized to placebo were switched to RINVOQ 15 mg or upadacitinib 30 mg QD in a blinded manner.
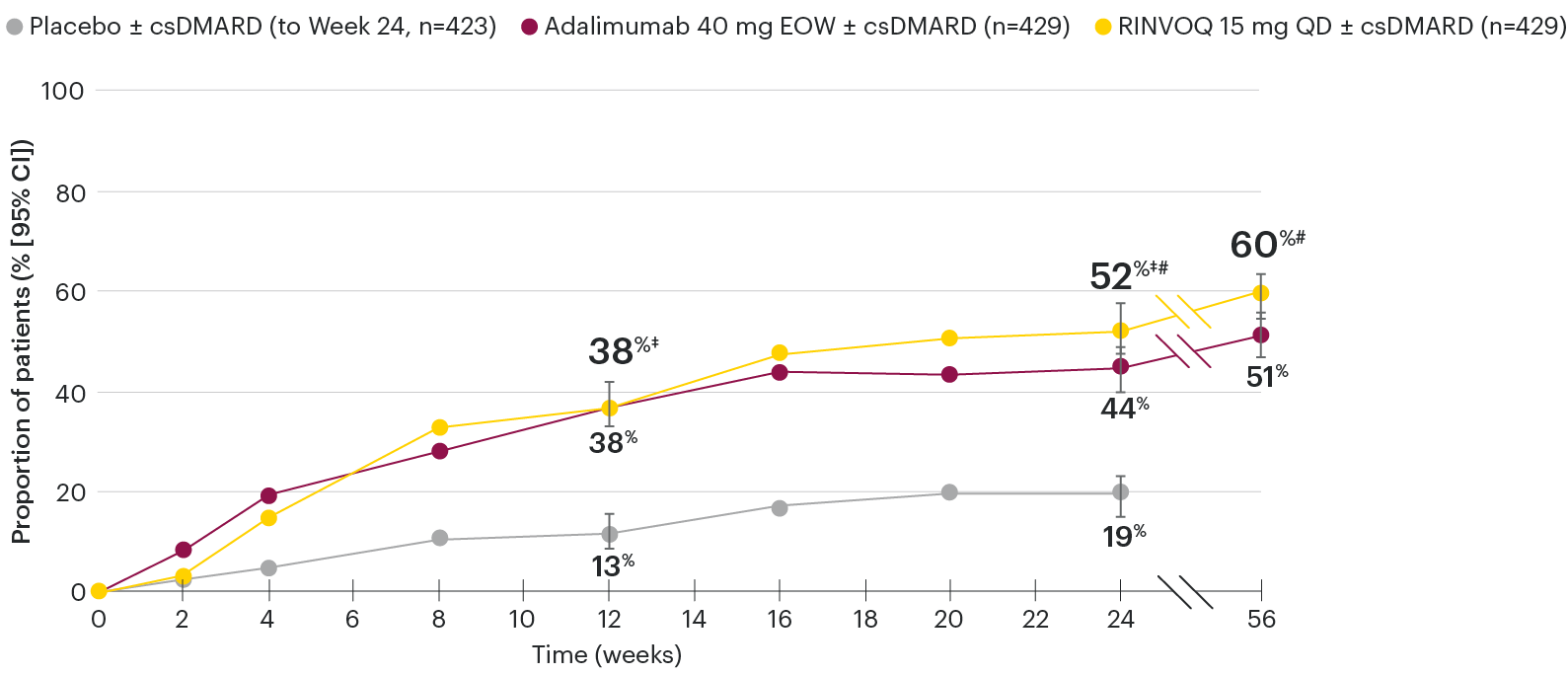
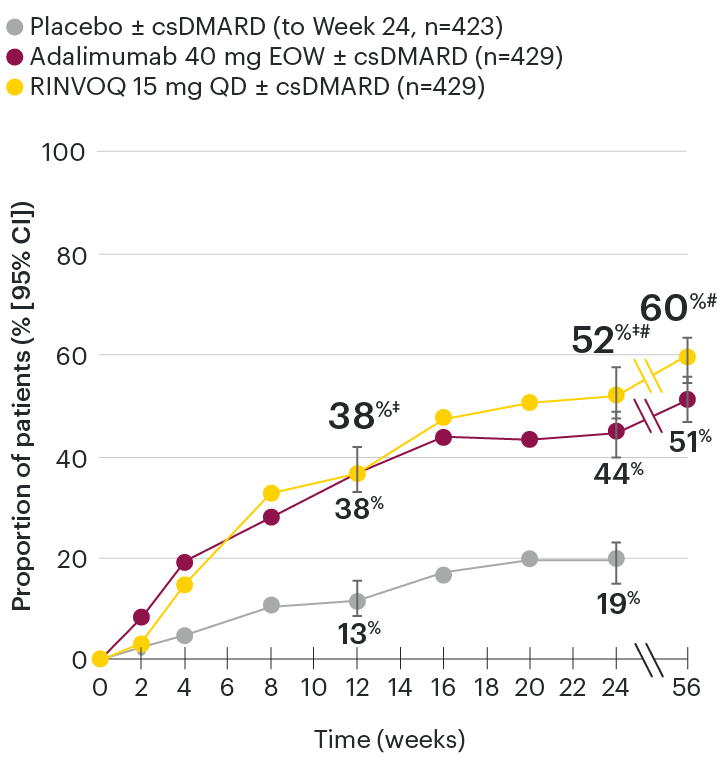
In patients with active PsA and an inadequate response to nonbiologic DMARDs
ACR50 response rates over time
SELECT-PsA 1: ACR50 response rates (NRI)1-4
‡Nominal P≤0.001 vs placebo.3
#Nominal P≤0.05 vs adalimumab.3,4
Nominal P-values denote data not controlled for multiplicity. No clinical inferences can be drawn.
At Week 24, all patients randomized to placebo were switched to RINVOQ 15 mg or upadacitinib 30 mg QD in a blinded manner.
Primary endpoint was the proportion of patients achieving ACR20 response with RINVOQ vs placebo at Week 12. ACR50 for RINVOQ vs placebo at Week 12 was an additional key secondary endpoint, not multiplicity-controlled. Comparison vs adalimumab was not prespecified.
In patients with active PsA and an inadequate response to nonbiologic DMARDs
ACR70 response rates over time
SELECT-PsA 1: ACR70 response rates (NRI)1-4
‡Nominal P≤0.001 vs placebo.3
#Nominal P≤0.05 vs adalimumab.3
¥Nominal P≤0.01 vs adalimumab.4
Nominal P-values denote data not controlled for multiplicity. No clinical inferences can be drawn.
At Week 24, all patients randomized to placebo were switched to RINVOQ 15 mg or upadacitinib 30 mg QD in a blinded manner.
Primary endpoint was the proportion of patients achieving ACR20 response with RINVOQ vs placebo at Week 12. ACR70 for RINVOQ vs placebo at Week 12 was an additional key secondary endpoint, not multiplicity-controlled. Comparison vs adalimumab was not prespecified.
DATA LIMITATIONS: Data not labeled as a ranked primary or secondary endpoint were prespecified, however they were not ranked, not controlled for multiplicity, and have nominal P-values; therefore, treatment differences could represent chance findings. No conclusions regarding these comparisons can be made.
95% confidence intervals are displayed as error bars in the chart. Missing data were handled using NRI.
ACR20/50/70: American College of Rheumatology 20%/50%/70% improvement in both tender and swollen joint counts, plus three of the following: patient assessments of pain, global disease activity and physical function, physician global assessment of disease activity and acute phase reactant (high sensitivity C-reactive protein); csDMARD: conventional synthetic disease-modifying antirheumatic drug; EOW: every other week; NRI: nonresponder imputation; QD: once daily.
In patients with active PsA and an inadequate response to nonbiologic DMARDs
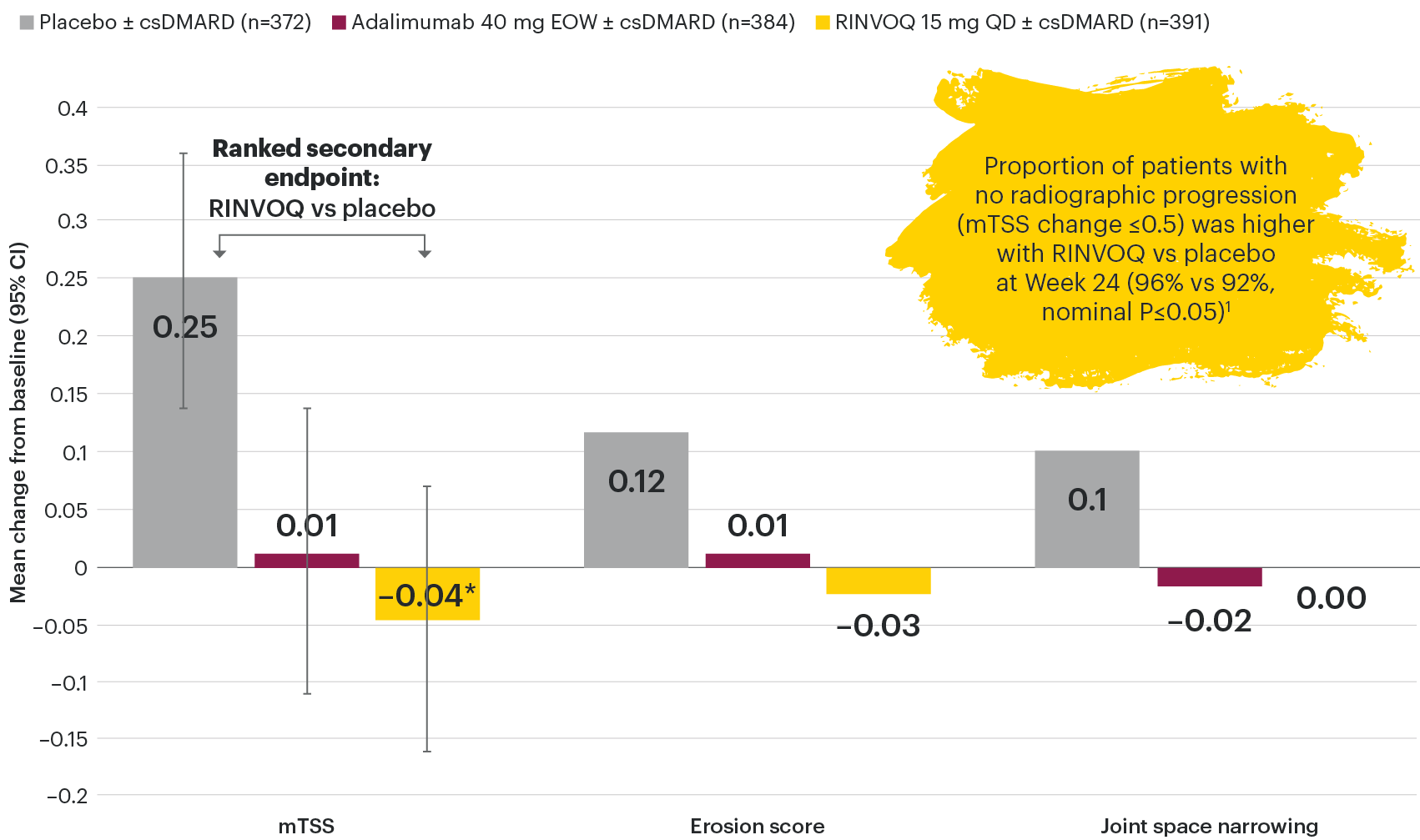
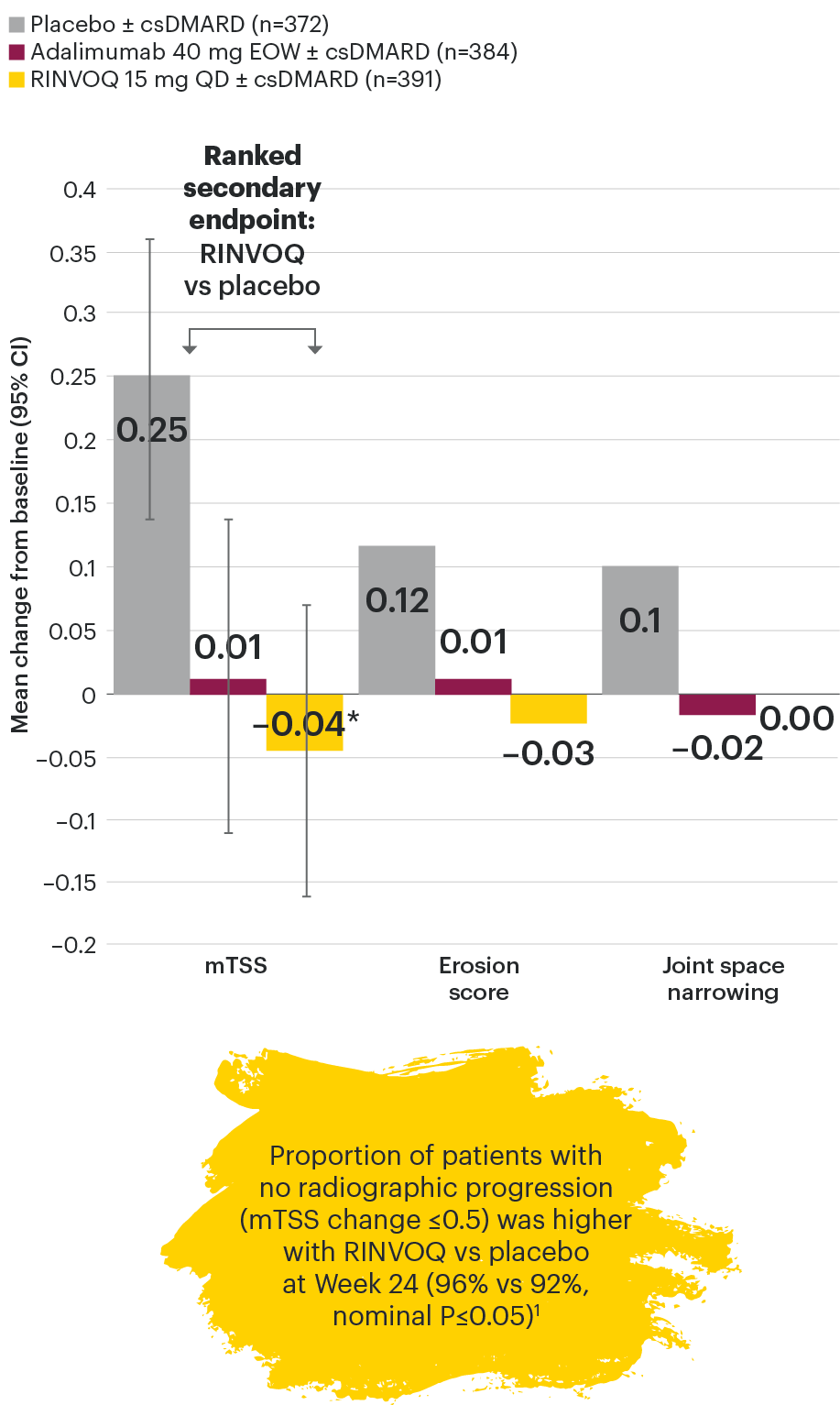
Joint protection vs placebo across radiographic endpoints
SELECT-PsA 1: Inhibition of structural joint damage progression at Week 24 (linear extrapolation)1,2
DATA LIMITATIONS: Data not labeled as a primary or ranked secondary endpoint were prespecified, however they were not ranked, not controlled for multiplicity, and have nominal P-values; therefore, treatment differences could represent chance findings. No conclusions regarding these comparisons can be made. No statistical comparisons were made between RINVOQ and adalimumab groups for radiographic endpoints.
95% confidence intervals are displayed as error bars in the chart.
csDMARD: conventional synthetic disease-modifying antirheumatic drug; EOW: every other week; mTSS: modified total Sharp score; QD: once daily.
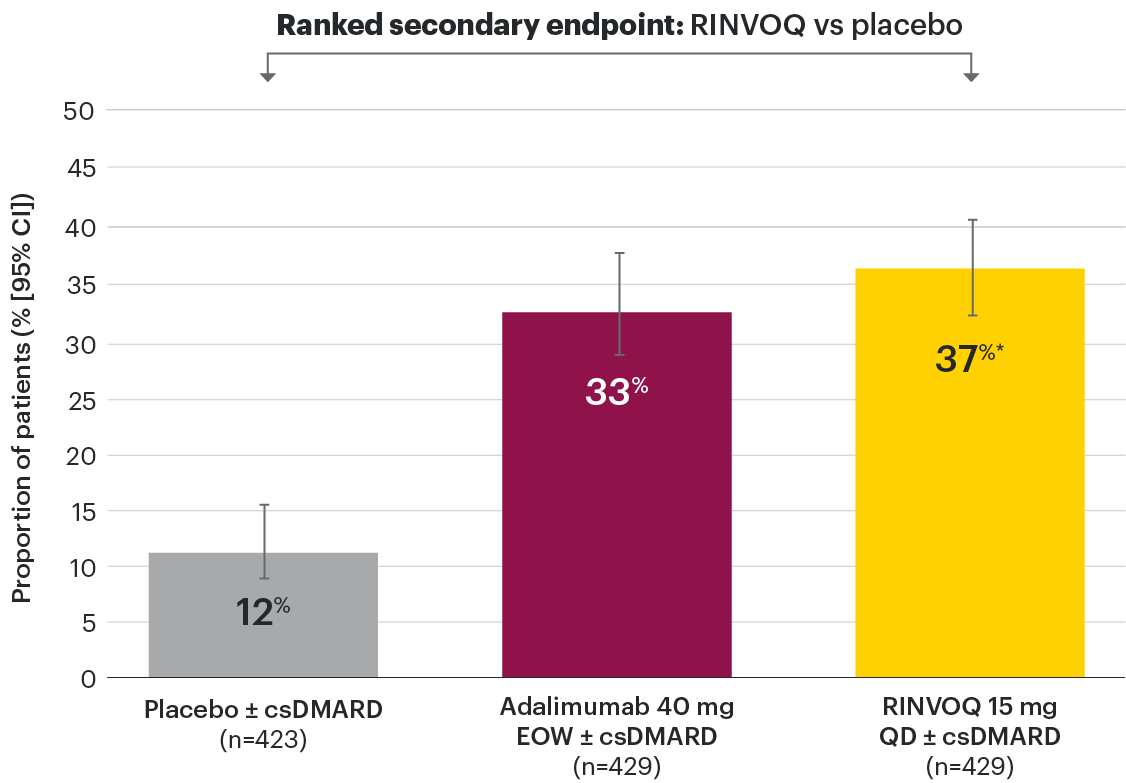
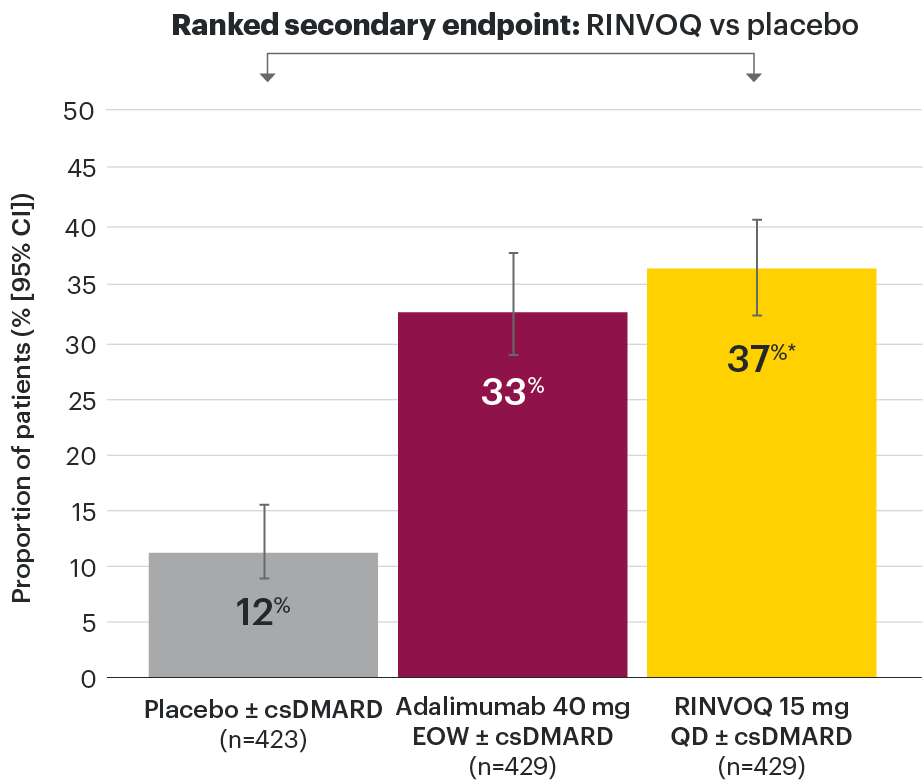
Efficacy across key PsA manifestations
In patients with active PsA and an inadequate response to nonbiologic DMARDs
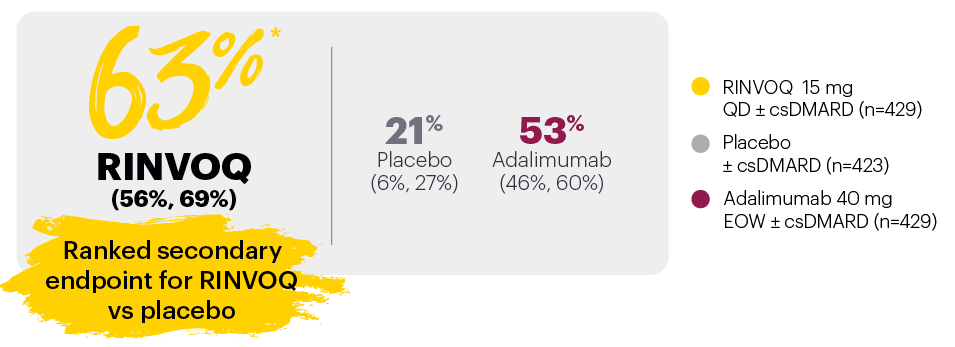
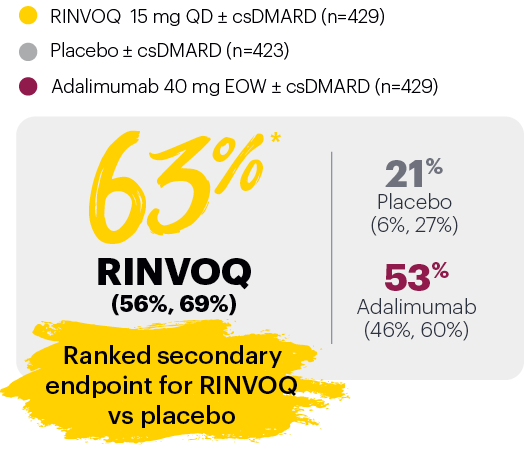
PASI 75 response week 16 (NRI)1,2
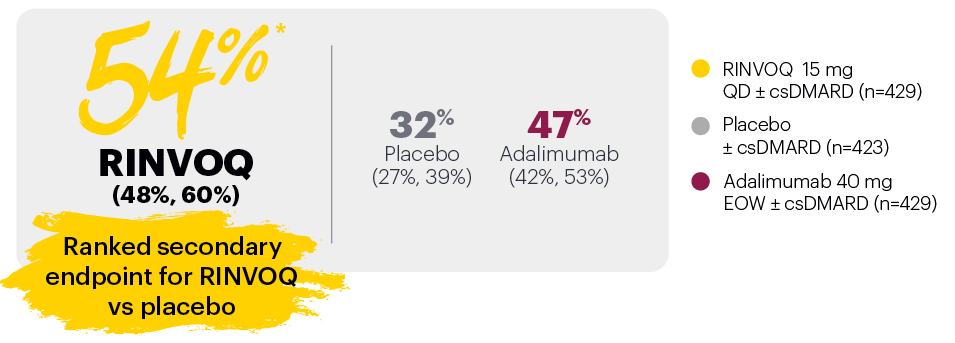
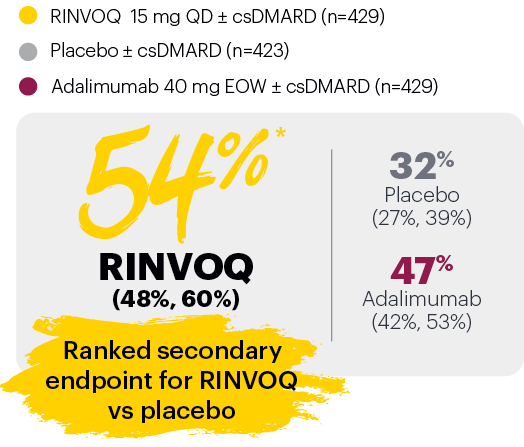
Resolution of dactylitis (LDI=0) at Week 24 (NRI)1,2,5
‡Nominal P≤0.001 vs placebo, not multiplicity-controlled. No clinical inferences can be drawn.
In subjects with baseline presence of dactylitis (LDl>0, n=126, 127, and 136, respectively, for placebo, adalimumab, and RINVOQ).
#RINVOQ 15 mg did not meet superiority vs adalimumab for ACR20 response, thus statistical significance vs placebo regarding proportion of patients achieving resolution of dactylitis could not be tested under the hierarchical analysis plan.
Subjects rescued at Week 16 were imputed as nonresponders in the resolution of enthesitis and dactylitis at Week 24.
DATA LIMITATIONS: Data not labeled as a ranked primary or secondary endpoint were prespecified, however they were not ranked, not controlled for multiplicity, and have nominal P-values; therefore, reatment differences could represent chance findings. No conclusions regarding these comparisons can be made.
The BASDAI is composed of 6 items investigating 5 domains (fatigue, spinal pain, joint pain/swelling, areas of localized tenderness, and morning stiffness).7
95% confidence intervals are included in parentheses after each percentage, where available.
Missing data were handled using NRI.
BSA: body surface area; csDMARD: conventional synthetic disease-modifying antirheumatic drug; EOW: every other week; LDI: Leeds Dactylitis Index; LEI: Leeds Enthesitis Index; NRI: nonresponder imputation; PASI 75: Psoriasis Area and Severity Index 75% improvement; QD: once daily.
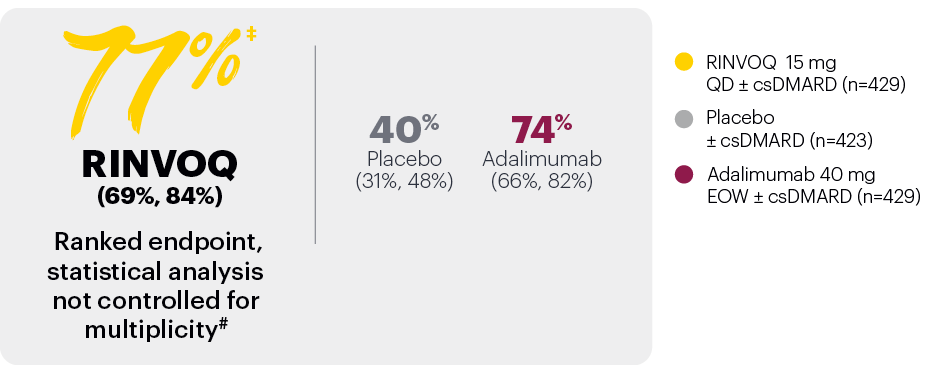
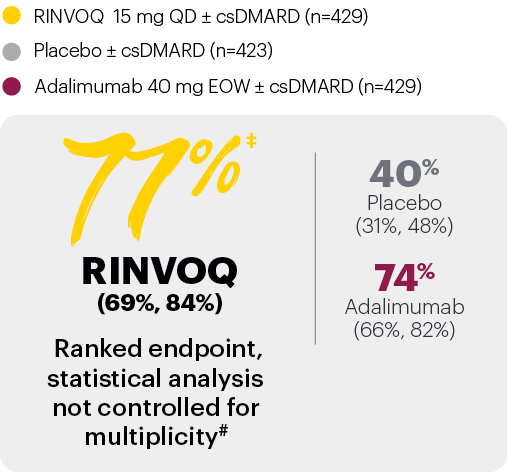
In patients with active PsA and an inadequate response to nonbiologic DMARDs
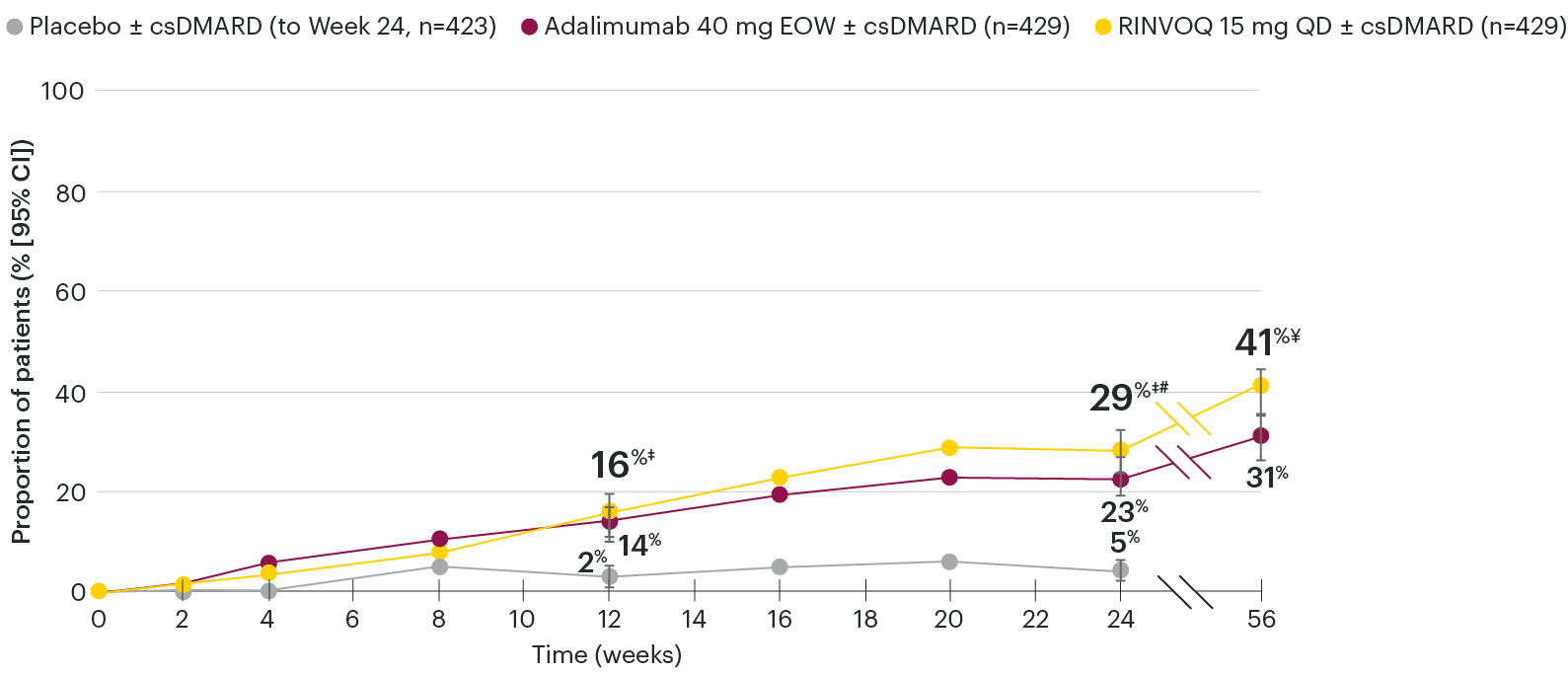
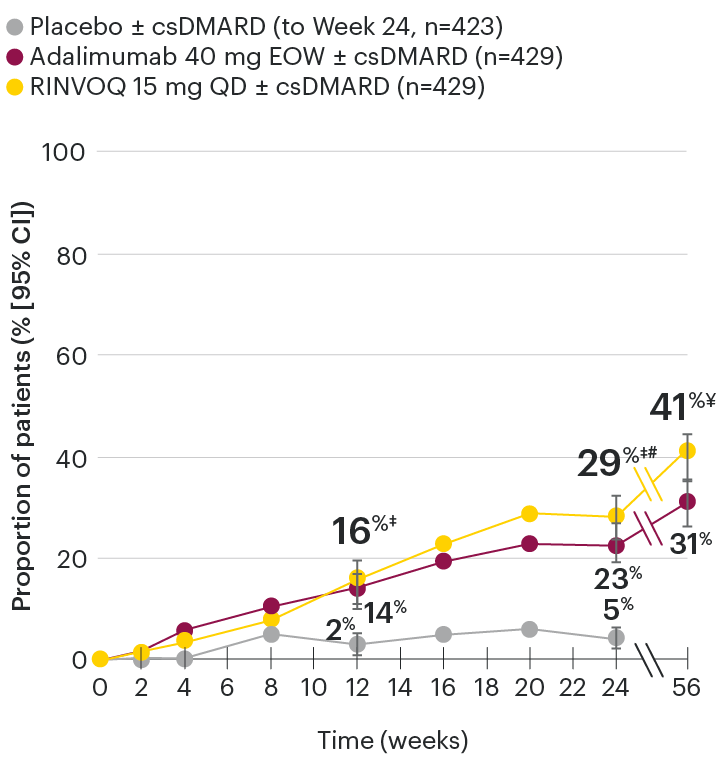
Significantly more patients treated with RINVOQ achieved MINIMAL DISEASE ACTIVITY† vs placebo at Week 24
SELECT-PsA 1: Minimal disease activity at Week 24 (NRI)1,2
*P≤0.001 vs placebo, statistically significant in the multiplicity-controlled analysis.
- Tender joint count ≤1
- Swollen joint count ≤1
- PASI ≤1 or BSA-Ps ≤3%
- Patient pain NRS ≤1.5
- Patient Global Disease Activity NRS ≤2
- HAQ-DI ≤0.5
- Tender entheseal points (LEI) ≤1
Subjects rescued at Week 16 were imputed as nonresponders in the MDA analysis.
95% confidence intervals are displayed as error bars in the chart.
Missing data were handled using NRI.
BSA-Ps: body surface area with psoriasis; csDMARD: conventional synthetic disease-modifying antirheumatic drug; EOW: every other week; HAQ-DI: Health Assessment Questionnaire Disability Index; LEI: Leeds Enthesitis Index; NRI: nonresponder imputation; NRS: numeric rating scale; PASI: Psoriasis Area and Severity Index; QD: once daily.
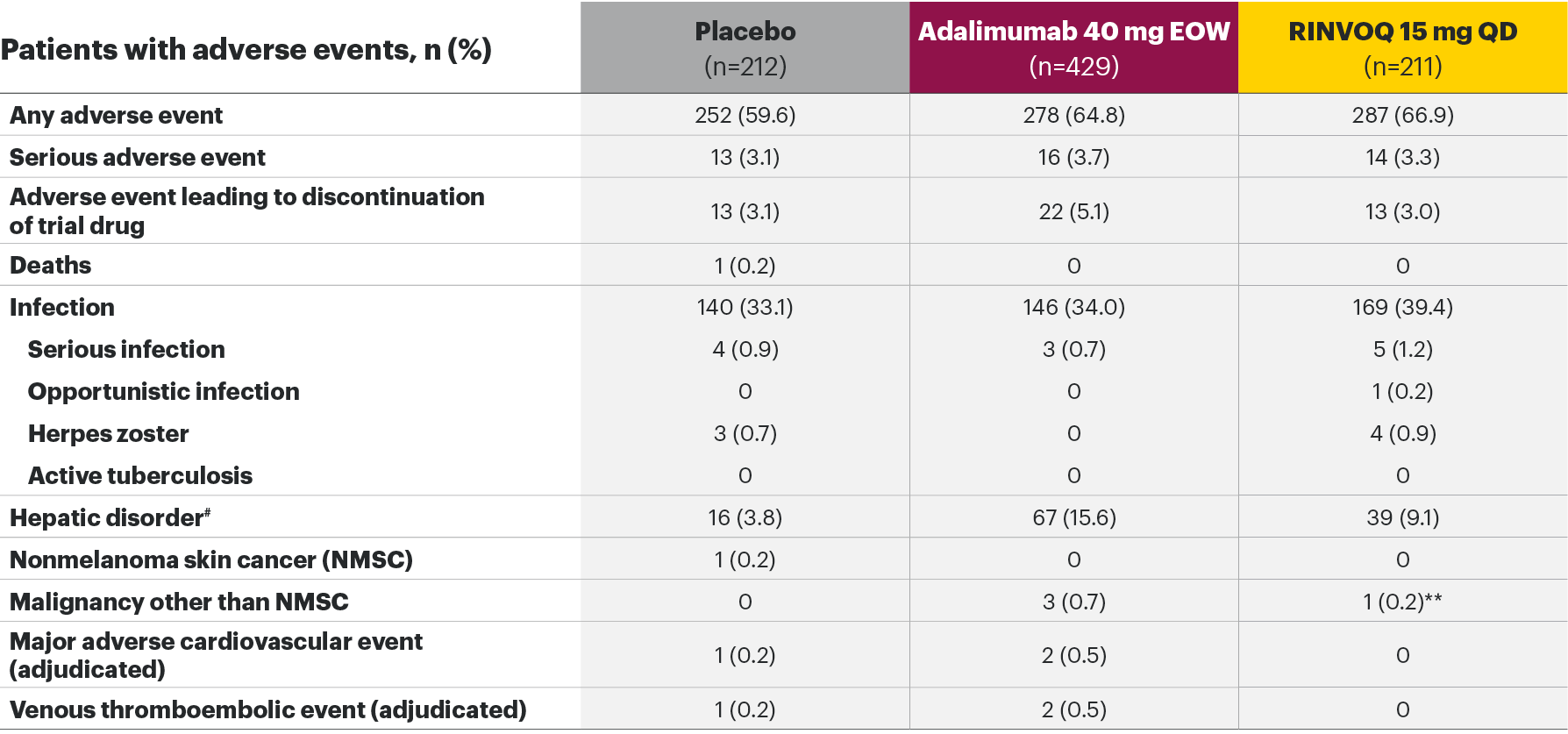
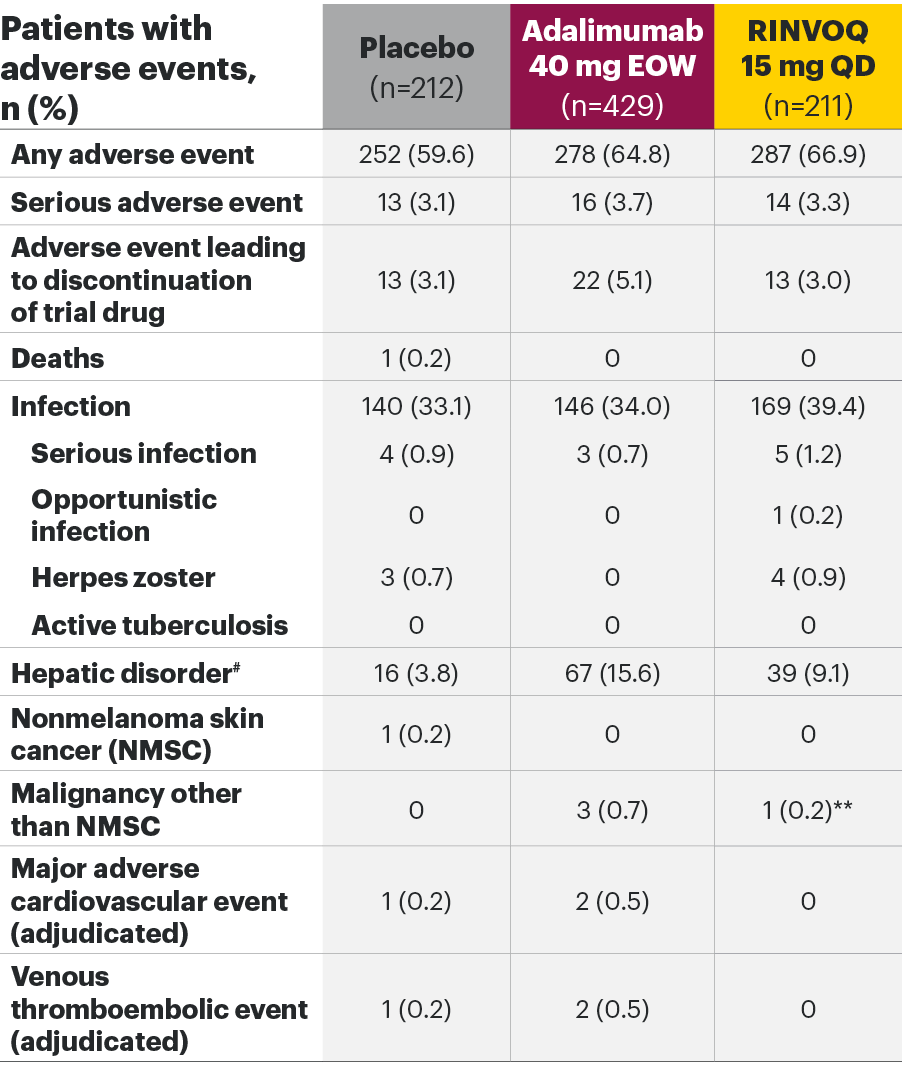
The safety profile of RINVOQ in PsA was consistent with previously reported results in RA1,2,4*
SELECT-PsA 1: Adverse events through Weeks 24 and 562,6
Most common adverse events: In SELECT-PsA 1, the most common AEs at Week 24 were upper respiratory tract infection, nasopharyngitis, blood CPK increase, ALT increase, and AST increase.2|| At Week 56, the most commonly reported AEs were upper respiratory tract infection and blood CPK elevations.4
For the safety analysis at Week 56, the RINVOQ 15-mg group included patients who were originally randomized to placebo and switched to RINVOQ 15 mg at Week 24.
*Higher incidences of acne and bronchitis were observed in patients treated with RINVOQ 15 mg (1.3% and 3.9%, respectively) compared to placebo (0.3% and 2.7%, respectively). In patients treated with RINVOQ in combination with methotrexate therapy compared to patients treated with monotherapy, higher rates of serious infections (2.6 E/100 PYs and 1.3 E/100 PYs, respectively) and hepatic transaminase elevations (ALT elevations Grade 3 and higher rates 1.4% and 0.4%, respectively) were observed. There was a higher rate of serious infections in patients ≥65 years of age, although data are limited.1
†Lung cancer metastasis.
‡Excluding tuberculosis and herpes zoster.
§There were 11 malignancies reported in the RINVOQ 15-mg group (4 basal cell carcinomas, 2 squamous cell carcinomas of skin, and one event each of endometrial adenocarcinoma, lung adenocarcinoma, lung cancer metastatic, malignant melanoma, and neuroendocrine carcinoma) and 6 malignancies reported with adalimumab (2 basal cell carcinomas, and one event each of colon cancer metastatic, ovarian cancer, pancreatic carcinoma metastatic, and uterine cancer).
||Treatment-emergent AEs reported in ≥5% of patients in any treatment arm through Week 24.
¶At Week 56, NMSC, malignancy other than NMSC, MACE, VTEs, and death were reported as exposure-adjusted incidence rates (EAIR). All other adverse events at Week 56 were reported as exposure-adjusted event rates.
#Hemoglobin: Grade 3 (<80); neutrophils: Grade 3 (0.5-<1.0), Grade 4 (<0.5); lymphocytes: Grade 3 (0.2-<0.5), Grade 4 (<0.2); platelets: Grade 3 (25-<50), Grade 4 (<25); alanine aminotransferase and aspartate aminotransferase: Grade 3 (>5.0-20.0xULN), Grade 4 (>20.0xULN); creatinine: Grade 3 (>3.0-6.0xULN or >3.0xbaseline), Grade 4 (>6.0xULN); creatine phosphokinase: Grade 3 (>5.0xULN-10.0xULN), Grade 4 (>10.0xULN).
AE: adverse event; ALT: alanine aminotransferase; EOW: every other week; QD: once daily; MACE: major adverse cardiovascular event; NMSC: nonmelanoma skin cancer; QD: once daily; ULN: upper limit of normal; VTE: venous thromboembolic event.
RINVOQ Important Safety Information1
Contraindications
RINVOQ is contraindicated in patients hypersensitive to the active substance or to any of the excipients, in patients with active tuberculosis (TB) or active serious infections, in patients with severe hepatic impairment, and during pregnancy.
Special warnings and precautions for use
Immunosuppressive medicinal products
Use in combination with other potent immunosuppressants is not recommended.
Serious infections
Serious and sometimes fatal infections have been reported in patients receiving upadacitinib. The most frequent serious infections reported included pneumonia and cellulitis. Cases of bacterial meningitis have been reported. Among opportunistic infections, TB, multidermatomal herpes zoster, oral/esophageal candidiasis, and cryptococcosis have been reported with upadacitinib. As there is a higher incidence of infections in patients ≥65 years of age, caution should be used when treating this population.
Viral reactivation
Viral reactivation, including cases of herpes zoster, was reported in clinical studies. The risk of herpes zoster appears to be higher in Japanese patients treated with upadacitinib.
Vaccinations
The use of live, attenuated vaccines during or immediately prior to therapy is not recommended. It is recommended that patients be brought up to date with all immunizations, including prophylactic zoster vaccinations, prior to initiating upadacitinib, in agreement with current immunization guidelines.
Malignancy
The risk of malignancies, including lymphoma is increased in patients with rheumatoid arthritis (RA). Malignancies, including nonmelanoma skin cancer (NMSC), have been reported in patients treated with upadacitinib. Consider the risks and benefits of upadacitinib treatment prior to initiating therapy in patients with a known malignancy other than a successfully treated NMSC or when considering continuing upadacitinib therapy in patients who develop a malignancy.
Hematological abnormalities
Treatment should not be initiated, or should be temporarily interrupted, in patients with hematological abnormalities observed during routine patient management.
Cardiovascular risk
RA patients have an increased risk for cardiovascular disorders. Patients treated with upadacitinib should have risk factors (e.g., hypertension, hyperlipidemia) managed as part of usual standard of care.
Lipids
Upadacitinib treatment was associated with dose-dependent increases in lipid parameters, including total cholesterol, low-density lipoprotein cholesterol, and high-density lipoprotein cholesterol.
Hepatic transaminase elevations
Treatment with upadacitinib was associated with an increased incidence of liver enzyme elevation compared to placebo.
Venous thromboembolisms
Events of deep vein thrombosis (DVT) and pulmonary embolism (PE) have been reported in patients receiving JAK inhibitors, including upadacitinib. Upadacitinib should be used with caution in patients at high risk for DVT/PE.
Adverse reactions
The most commonly reported adverse reactions in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis clinical trials (≥2% of patients in at least one of the indications) with upadacitinib 15 mg were upper respiratory tract infections, blood creatine phosphokinase (CPK) increased, alanine transaminase (ALT) increased, bronchitis, nausea, cough, aspartate transaminase (AST) increased, and hypercholesterolemia. The most common serious adverse reactions were serious infections.
The safety profile of upadacitinib with long term treatment was generally similar to the safety profile during the placebo-controlled period across indications.
Overall, the safety profile observed in patients with psoriatic arthritis or active ankylosing spondylitis treated with upadacitinib 15 mg was consistent with the safety profile observed in patients with RA.
This is not a complete summary of all safety information.
Please see the RINVOQ Summary of Product Characteristics for complete prescribing information.
[Insert local HUMIRA Important Safety Information]
Please see the HUMIRA Summary of Product Characteristics for complete prescribing information.
NOTE TO AFFILIATES: Consult local MRL regarding adding a link to the adalimumab SmPC in the main navigation area.
- RRINVOQ [Summary of Product Characteristics]. AbbVie Deutschland GmbH & Co. KG.
- McInnes IB, Anderson JK, Magrey M, et al. Trial of upadacitinib and adalimumab for psoriatic arthritis. N Engl J Med. 2021;384(13):1227-1239. doi:10.1056/NEJMoa2022516
- AbbVie Data on File. ABVRRTI71394 [Week 24 data].
- McInnes I, Magrey M, Merola J, et al. Upadacitinib in patients with psoriatic arthritis and an inadequate response to non-biologic therapy: 56-week data from the Phase 3 SELECT-PsA 1 study. Ann Rheum Dis. 2021 [Draft publication].
- AbbVie Data on File. ABVRRTI71396.
- European Medicines Agency (EMA). RINVOQ assessment report - variation. https://www.ema.europa.eu/en/documents/variation-report/rinvoq-h-c-004760-ii-0004-epar-assessmentreportvariation_en.pdf. Updated March 2021. Accessed May 10, 2021.
- Zochling J. Measures of symptoms and disease status in ankylosing spondylitis: Ankylosing Spondylitis Disease Activity Score (ASDAS), Ankylosing Spondylitis Quality of Life Scale (ASQoL), Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), Bath Ankylosing Spondylitis Functional Index (BASFI), Bath Ankylosing Spondylitis Global Score (BAS-G), Bath Ankylosing Spondylitis Metrology Index (BASMI), Dougados Functional Index (DFI), and Health Assessment Questionnaire for the Spondylarthropathies (HAQ-S). Arthritis Care Res (Hoboken). 2011;63(suppl 11):S47-S58.
- Coates LC, Fransen J, Helliwell PS. Defining minimal disease activity in psoriatic arthritis: a proposed objective target for treatment. Ann Rheum Dis. 2010;69(1):48-53. doi:10.1136/ard.2008.102053