Note to Affiliate: The term reliable must be used in conjunction with the dosing schedule. Please evaluate the use of this term within your own country-specific regulations.
RELIABLE DOSING SCHEDULE WITH
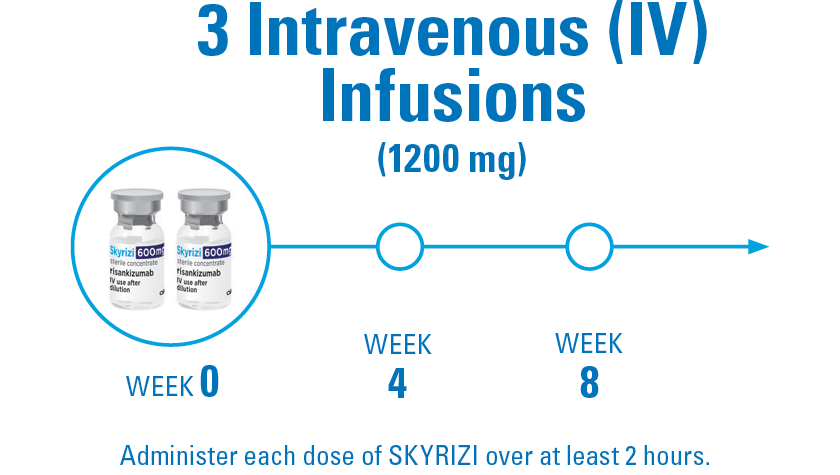
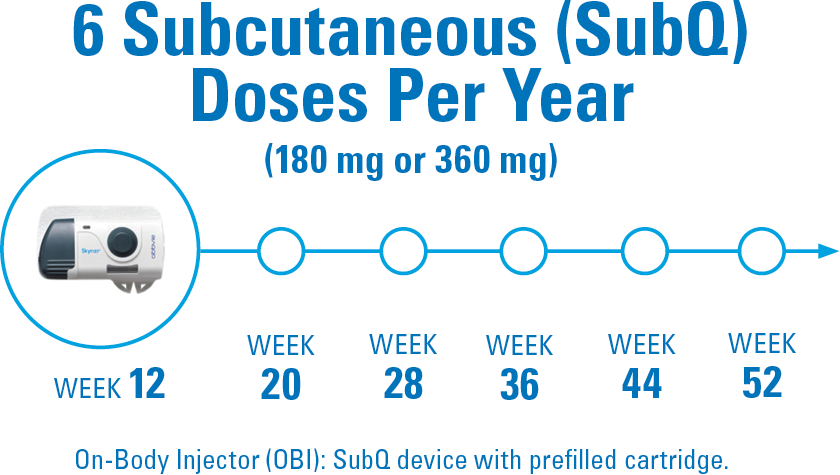
6 DOSES PER YEAR AFTER INDUCTION
DOSING CONSIDERATIONS: The recommended dose is 1200 mg administered by IV infusion at Week 0, Week 4, and Week 8, followed by 180 mg or 360 mg administered by SubQ injection at Week 12, and every 8 weeks thereafter. Consideration should be given to discontinuing treatment in patients who have shown no evidence of therapeutic benefit by Week 24. SKYRIZI is intended for use under the guidance and supervision of a healthcare professional. Patients may self-inject SKYRIZI after training in use of the OBI. Provide proper training to patients and/or caregivers on the use of the SKYRIZI OBI according to the Instructions for Use.1
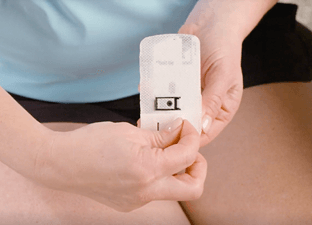
THE ON-BODY INJECTOR (OBI)
A NEW TECHNOLOGY FOR UC PATIENTS
The OBI gives patients the ability to administer the required maintenance dose at home, in up to 5 minutes1
DOSING CONSIDERATIONS: SKYRIZI is intended for use under the guidance and supervision of a healthcare professional. Patients may self-inject SKYRIZI after training in use of the OBI. Provide proper training to patients and/or caregivers on the use of the SKYRIZI OBI according to the Instructions for Use.1
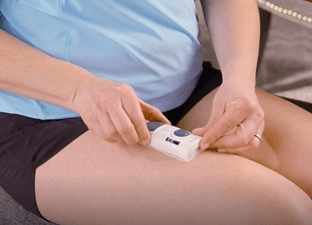
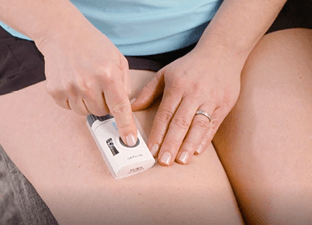
PREPARATION AND ADMINISTRATION OF THE
OBI MAINTENANCE DOSING IN 5 STEPS
Help your patients remember these 5 steps1
See the SKYRIZI Instructions for Use to get the details on preparing and injecting SKYRIZI.
The SKYRIZI Instructions for Use includes the full set of detailed instructions on preparation and administration of SKYRIZI. Encourage the patient to read the instructions before administration.
For healthcare professionals. Please see Summary of Product Characteristics, including Instructions for Use.
Indication1
SKYRIZI is indicated for the treatment of adult patients with moderately to severely active ulcerative colitis who have had an inadequate response to, lost response to, or were intolerant to conventional therapy or a biologic therapy.
Important Safety Information1
Affiliate to insert local ISI.
Risankizumab is contraindicated in patients hypersensitive to the active substance or to any of the excipients, and in patients with clinically important active infections (e.g. active tuberculosis).
Risankizumab may increase the risk of infection. In patients with a chronic infection, a history of recurrent infection, or known risk factors for infection, risankizumab should be used with caution. Treatment with risankizumab should not be initiated in patients with any clinically important active infection until the infection resolves or is adequately treated.
Patients treated with risankizumab should be instructed to seek medical advice if signs or symptoms of clinically important chronic or acute infection occur. If a patient develops such an infection or is not responding to standard therapy for the infection, the patient should be closely monitored and risankizumab should not be administered until the infection resolves.
Prior to initiating treatment with risankizumab, patients should be evaluated for tuberculosis (TB) infection. Patients receiving risankizumab should be monitored for signs and symptoms of active TB. Anti-TB therapy should be considered prior to initiating risankizumab in patients with a past history of latent or active TB in whom an adequate course of treatment cannot be confirmed.
Prior to initiating therapy with risankizumab, completion of all appropriate immunisations should be considered according to current immunisation guidelines. If a patient has received live vaccination (viral or bacterial), it is recommended to wait at least 4 weeks prior to starting treatment with risankizumab. Patients treated with risankizumab should not receive live vaccines during treatment and for at least 21 weeks after treatment.
Serious hypersensitivity reactions, including anaphylaxis, have been reported with use of risankizumab. If a serious hypersensitivity reaction occurs, administration of risankizumab should be discontinued immediately and appropriate therapy initiated.
The most frequently reported adverse reactions were upper respiratory infections (13% in psoriasis, 15.6% in Crohn’s disease and 26.2% in ulcerative colitis).
Commonly (≥ 1/100 to < 1/10) reported adverse reactions included tinea infections, headache, pruritus, rash, eczema, fatigue, and injection site reactions.
This is not a complete summary of all safety information.
See SKYRIZI full Summary of Product Characteristics (SmPC) at www.ema.europa.eu
Globally, prescribing information varies; refer to the individual country product label for complete information.
IV: intravenous; JAK: Janus kinase; OBI: On-Body Injector; SubQ: subcutaneous.
Reference: 1. SKYRIZI [Summary of Product Characteristics]. AbbVie Deutschland GmbH & Co. KG; [DRAFT].