RINVOQ® (upadacitinib) is indicated for the treatment of adult patients with moderately to severely active ulcerative colitis (UC) who have had an inadequate response, lost response or were intolerant to either conventional therapy or a biologic agent.1
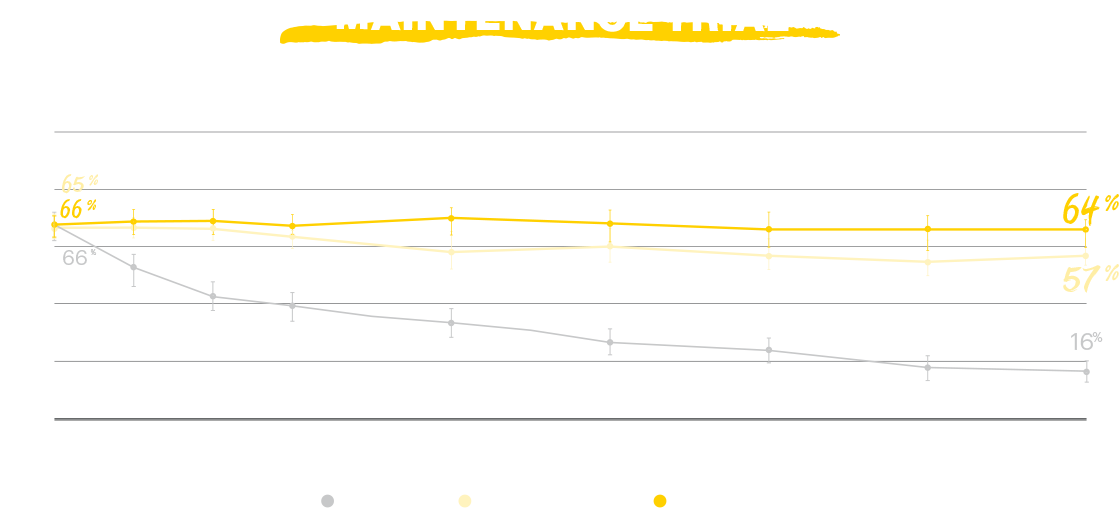
RINVOQ achieved the primary endpoints of clinical remission per adapted Mayo score at Induction Week 8 and Maintenance Week 521,2
▼ This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions.
A Phase 3 trial program involving 3 studies: 2 replicate induction studies (U-ACHIEVE Induction and U-ACCOMPLISH) and 1 maintenance study (U-ACHIEVE Maintenance). A total of 988 patients with moderately to severely active UC evaluating RINVOQ 45 mg QD vs placebo for induction and RINVOQ 15 mg QD and 30 mg QD vs placebo for maintenance treatment (N=451).1*
*Patients who achieved clinical response per adapted Mayo score with 8-week RINVOQ 45 mg QD induction treatment entered maintenance.
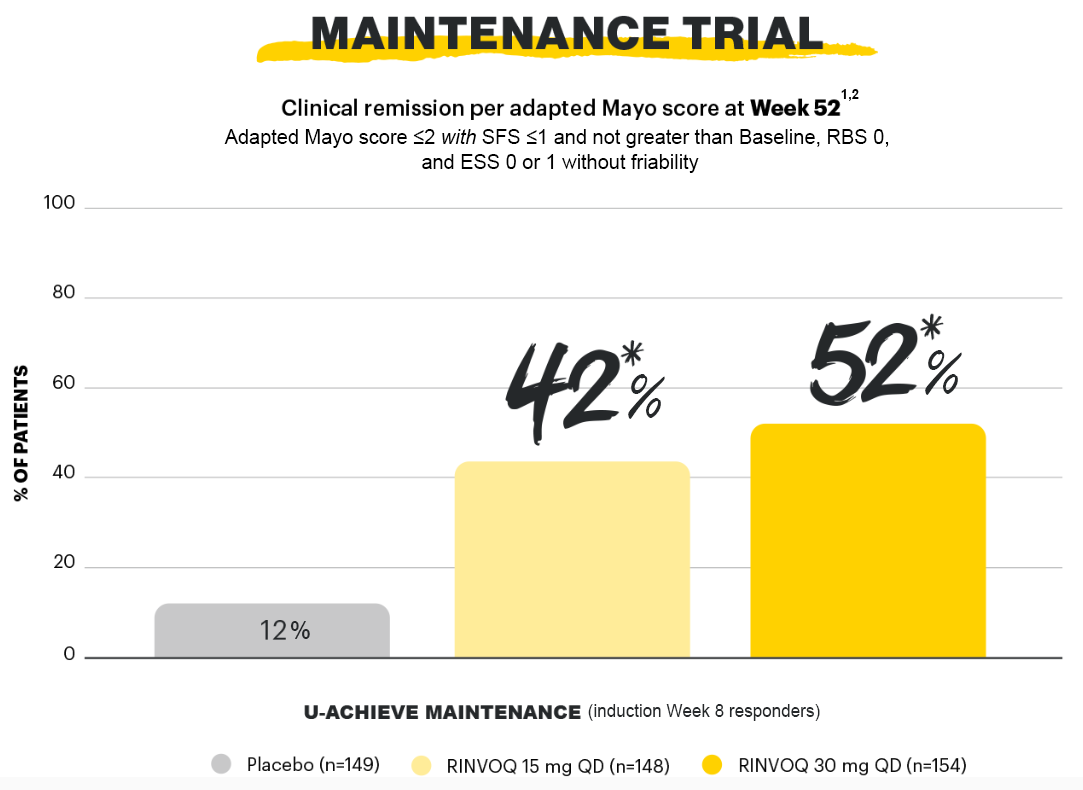
Proportion of patients with clinical remission per aMs at Week 52 (stool frequency subscore ≤1 and not greater than baseline plus rectal bleeding subscore=0 plus endoscopic subscore 0 or 1 without friability)1,2
*p<0.0001 vs placebo, multiplicity-controlled analysis (ITT).
Clinical remission per adapted Mayo score at Week 52 was the primary endpoint.1,2
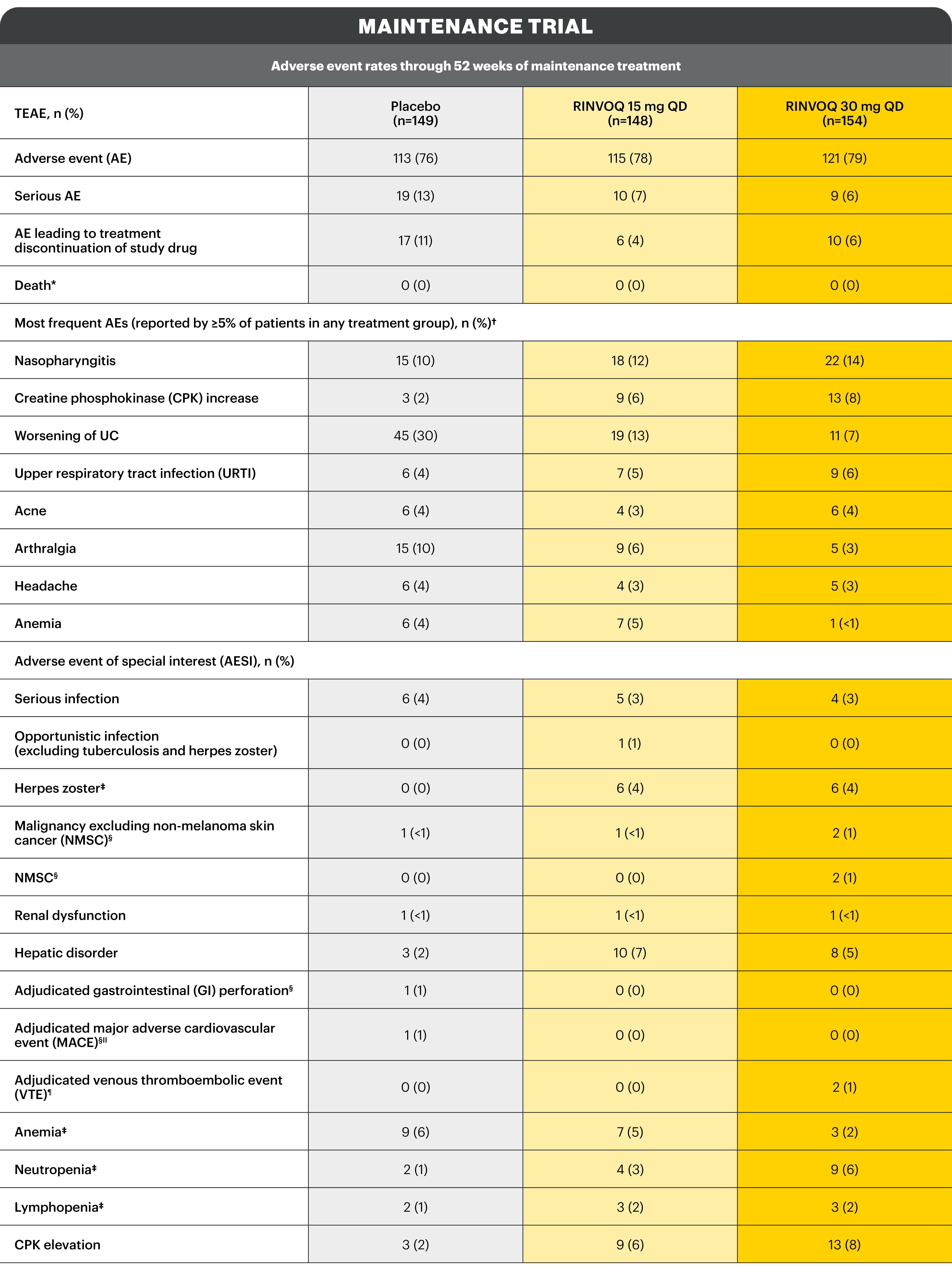
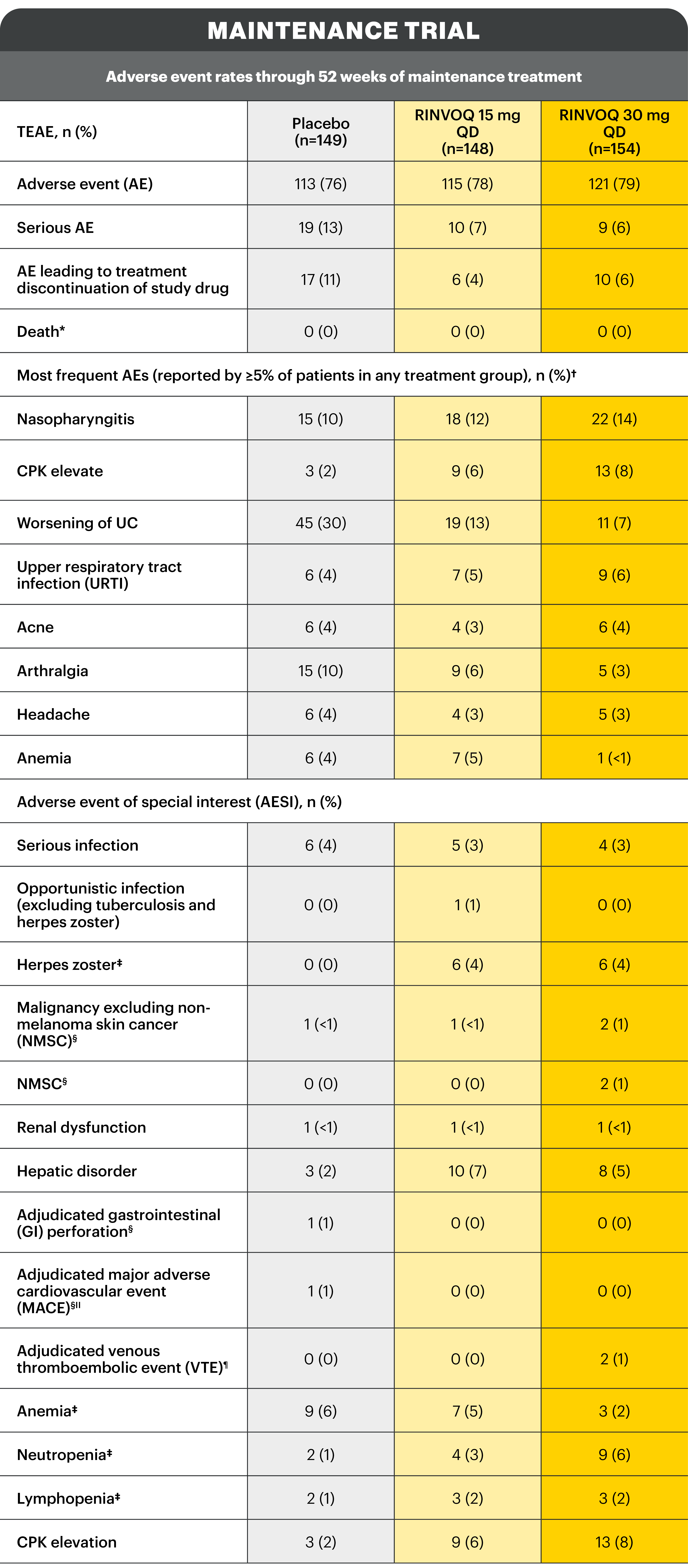
*Includes non-treatment emergent deaths. †The rates of the 4 most frequent adverse events are listed for all studies. ‡Search criteria were based on Company MedDRA Query. §These events were determined on the basis of external adjudication. IIMACE is defined as cardiovascular death, non-fatal myocardial infarction, and non-fatal stroke. ¶VTE is defined as deep vein thrombosis ad pulmonary embolism (fatal and non-fatal).
There were no AESIs of active tuberculosis or lymphoma in the study.2
AE: adverse event; AESI: adverse event of special interest; aMs: adapted Mayo score; CI: confidence interval; CPK: creatine phosphokinase; ESS: endoscopic subscore; GI: gastrointestinal; ITT: intention to treat; MACE: major adverse cardiac event; MedDRA: Medical Dictionary for Regulatory Activities; NMSC: non-melanoma skin cancer; NRI-C: non-responder imputation incorporating multiple imputations to handle missing data due to coronavirus disease 2019 (COVID-19); paMs: partial adapted Mayo score; pMs: partial Mayo score; QD: once daily; RBS: rectal bleeding score; TEAE: treatment-emergent adverse event; UC: ulcerative colitis; URTI: upper respiratory tract infection; VTE: venous thromboembolism.
Study designs: U-ACHIEVE Induction (UC-1) and U-ACCOMPLISH (UC-2) were replicate induction studies, both of which were multicenter, double-blind, placebo-controlled clinical studies. In UC-1 and UC-2, 988 patients (473 and 515 patients, respectively) were randomized to RINVOQ 45 mg QD or placebo for 8 weeks with a 2:1 treatment allocation ratio and included in the efficacy analysis. All enrolled patients had moderately to severely active UC defined as aMs of 5 to 9 with an ESS of 2 or 3 and demonstrated prior treatment failure including inadequate response, loss of response, or intolerance to prior conventional and/or biologic treatment. U-ACHIEVE Maintenance (UC-3) was a multicenter, double-blind, placebo-controlled clinical study with 451 patients who achieved clinical response per aMs (decrease ≥2 points and ≥30% from baseline and a decrease in RBS ≥1 from baseline or an absolute RBS ≤1) with 8-week RINVOQ 45 mg QD induction treatment. Patients were rerandomized 1:1:1 to receive either RINVOQ 15 mg QD, 30 mg QD, or placebo.1,2
Clinical remission per aMs (primary endpoint): SFS ≤1 and not greater than baseline, RBS=0, ESS 0 or 1 without friability. Clinical response per paMs (symptomatic response; secondary, multiplicity-controlled endpoint): decrease in paMs ≥1 point and ≥30% from baseline, PLUS a decrease in rectal bleeding score ≥1 or an absolute rectal bleeding score ≤1.1,2
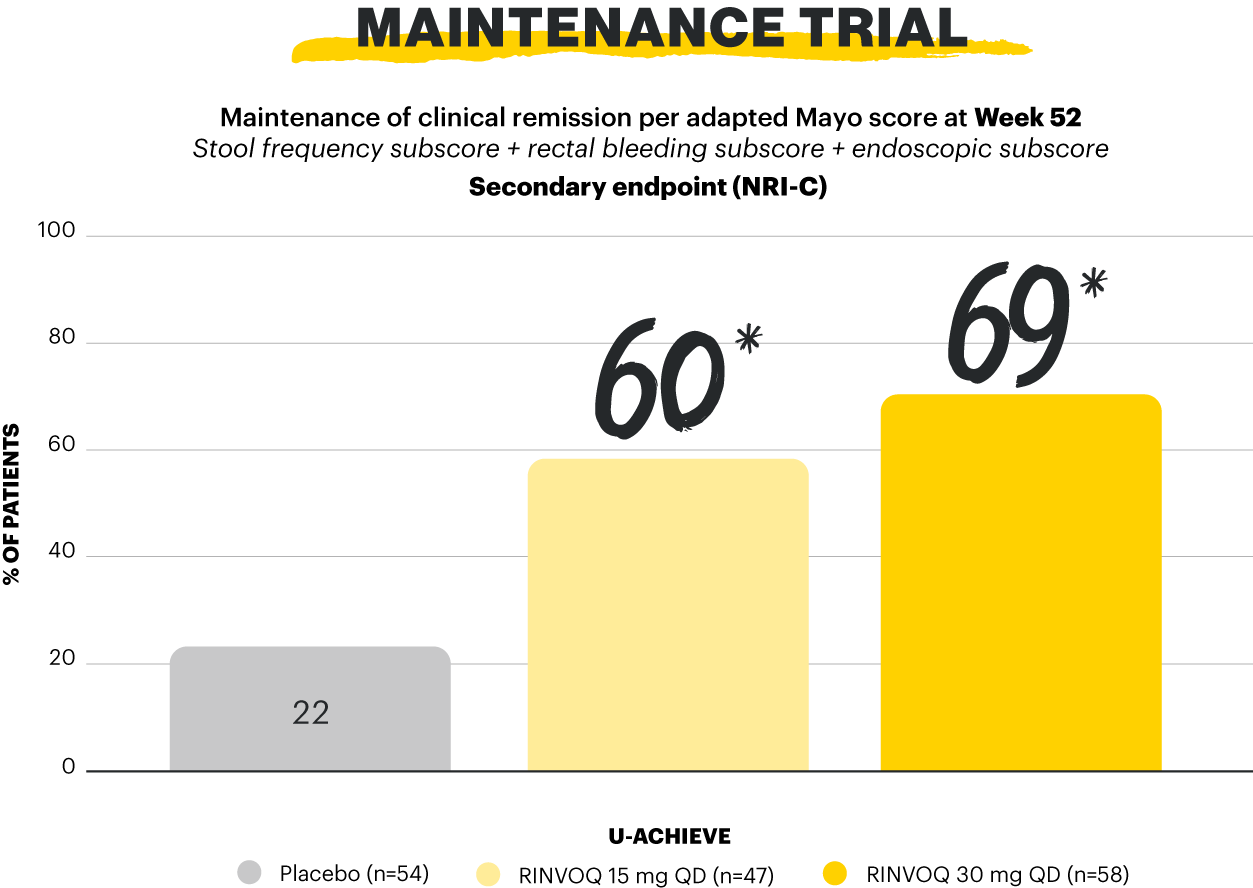
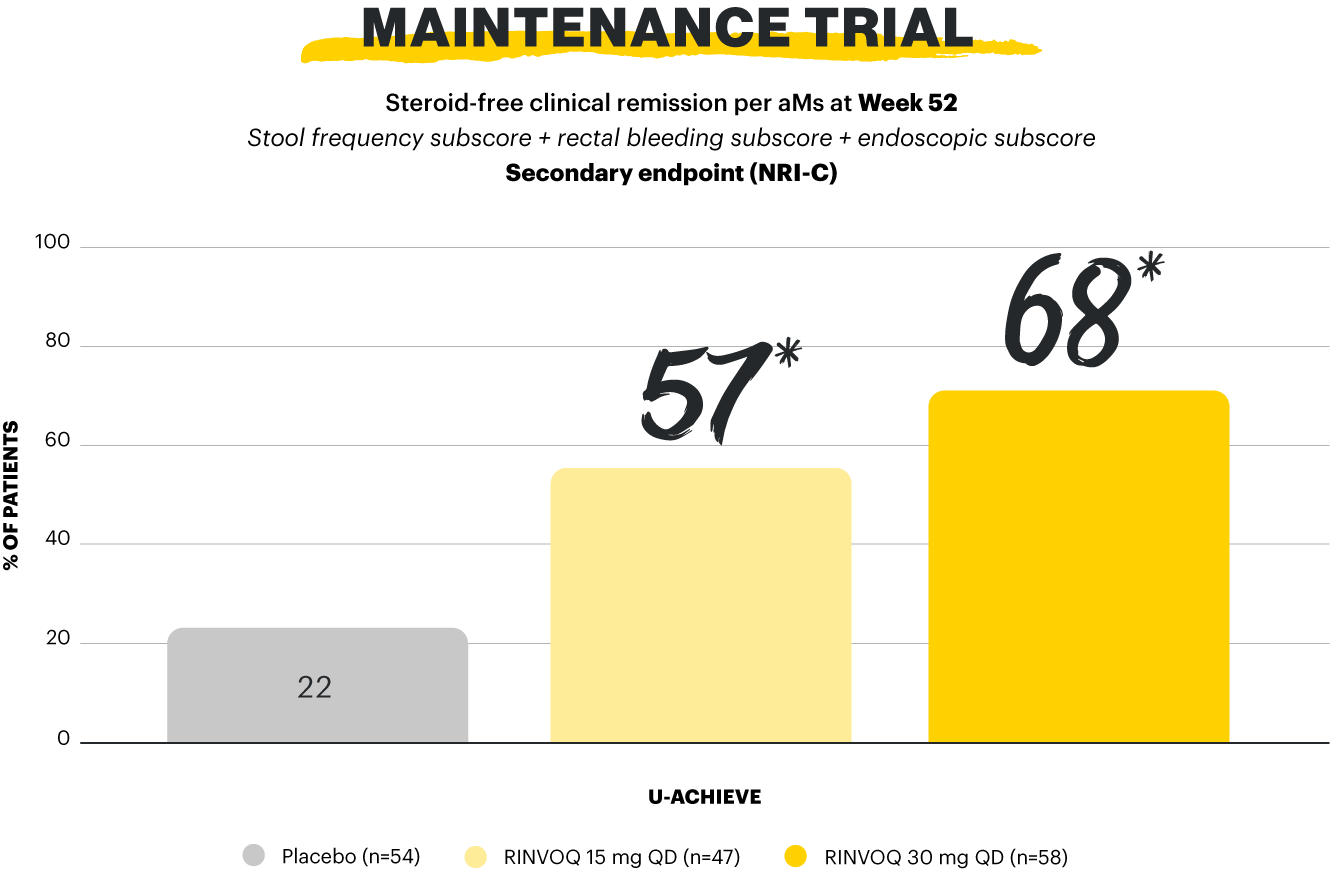
Clinical remission per pMs: pMS ≤2 with no subscore >1; steroid-free clinical remission per aMs: clinical remission per aMs at the end of 8-week RINVOQ 45 mg QD induction treatment (n=159) and at Week 52 of maintenance, and steroid-free ≥90 days immediately preceding Week 52.1,2
UP NEXT
RINVOQ is an oral, once daily, selective and reversible JAK inhibitor now approved for the treatment of adult patients with moderately to severely active ulcerative colitis who have had an inadequate response, lost response or were intolerant to either conventional therapy or a biologic agent.1
RINVOQ can be taken at any time of the day, with or without food. Tablets should be swallowed whole and should not be split, crushed, or chewed in order to ensure the entire dose is delivered correctly.1
A Phase 3 clinical trial program involving 3 studies: 2 replicate 8-week induction studies and 1 52-week maintenance study evaluated RINVOQ 45 mg QD vs placebo for induction and RINVOQ 15 mg QD and 30 mg QD vs placebo for maintenance treatment.1,2
[Please insert local summary of safety]
REFERENCES
- RINVOQ [Summary of Product Characteristics]. AbbVie Deutschland GmbH & Co. KG; December 2023.
- Danese S, Vermeire S, Zhou W, et al. Upadacitinib as induction and maintenance therapy for moderately to severely active ulcerative colitis: results from three phase 3, multicentre, double-blind, randomised trials. Lancet. 2022;399(10341):2113-2128. doi:10.1016/S0140-6736(22)00581-5
- Vermeire S, Colombel JF, Takeuchi K, et al. Upadacitinib therapy reduces ulcerative colitis symptoms as early as day 1. J Crohns Colitis. 2022;16(suppl 1):i087-i088. doi:10.1093/ecco-jcc/jjab232.077
- Srinivas TR, Bing H, Kang J, et al. Post hoc analyses: after the fact. Transplantation. 2015;5(99):17-20. doi:10.1097/TP.0000000000000581
- Dmitrienko A, D'Agostino RB Sr. Editorial: multiplicity issues in clinical trials. Stat Med. 2017;36(28):4423-4426. doi:10.1002/sim.7506