DRAFT FOR INTERNAL USE ONLY
This DRAFT of the Global RINVOQ CD AbbVie Pro (v0.2) has been developed based on the DRAFT RINVOQ CD CLM, ALL-RNQG-220127 (v1.0).
RINVOQ CD was approved by the European Commission in April 2023 and the DRAFT CLM is being updated accordingly but is not final approved. When implemented locally, affiliates must align AbbVie Pro to the forthcoming final approved RINVOQ CD CLM, ALL-RNQG-220127 (version to be determined).
Note to Affiliates: Please evaluate use of ALL claims, graphs/tables, and corresponding references (e.g., data on file, abstracts, posters, manuscripts) according to local standards, codes, and regulations.
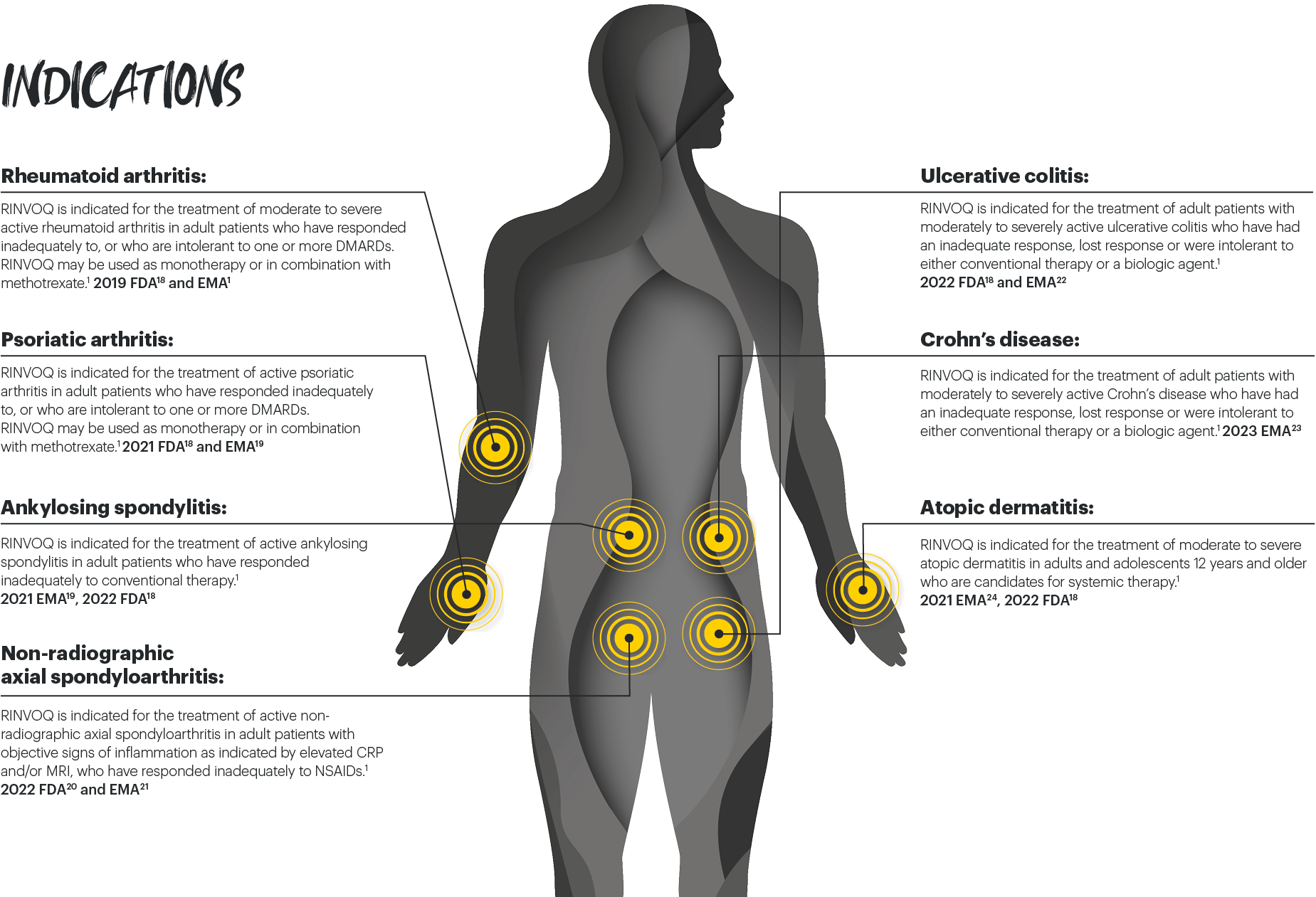
RINVOQ® (upadacitinib) is indicated for the treatment of adult patients with moderately to severely active Crohnʼs disease (CD) who have had an inadequate response, lost response or were intolerant to either conventional therapy or a biologic agent.1
RINVOQ achieved its co-primary endpoints of endoscopic response and clinical remission at Week 12 (Induction) and Week 52 (Maintenance)1
▼ This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions.
A Phase 3 trial program involving 3 studies:1
2 induction studies (U-EXCEL and U-EXCEED) and 1 maintenance study (U-ENDURE). 1,021 moderately to severely active CD patients evaluating RINVOQ 45 mg QD vs placebo for induction, and RINVOQ 15 mg QD or 30 mg QD vs placebo for maintenance treatment (N=502).1*
*Patients who achieved clinical response (≥30% decrease in average daily stool frequency and/or in abdominal pain score, neither worse than baseline) to 12 weeks of RINVOQ 45 mg QD induction treatment entered maintenance.
RINVOQ EXPERIENCE ACROSS RHEUMATOLOGY, DERMATOLOGY & GASTROENTEROLOGY
[Affiliates to update assets below with latest data on file]
*Beginning in RA. First patients dosed December 2015 in a RA Phase 3 clinical trial.
†103,127 RA patients, 17,942 AS and PsA patients, and 16,980 AD patients taking RINVOQ globally. Global patient numbers include OUS+US. Numbers are subject to change based on local market data adjustments.
‡Total patient number was calculated from adding the number of patients randomized in 20 trials. 13,557 patients includes all patients across all arms (active treatment and placebo) in the following Phase 3 trials in RA: (SELECT-EARLY, SELECT-NEXT, SELECT-COMPARE, SELECT-MONOTHERAPY, SELECT-BEYOND, SELECT-CHOICE), PsA (SELECT-PsA 1, SELECT-PsA 2), AS (SELECT-AXIS 1, SELECT-AXIS 2), PsA (SELECT-AXIS 2), AD (MEASURE UP 1, MEASURE UP 2, AD UP, HEADS UP), UC (U-ACHIEVE [UC-1], U-ACCOMPLISH [UC-2]), and CD (U-EXCEED [CD-1] and U-EXCEL [CD-2]). 7,932 patients were randomized to receive RINVOQ at any dose. Patients randomized to RINVOQ include 344 adolescent patients with moderate to severe AD not adequately controlled by topical medication(s).
AD: atopic dermatitis; APS: abdominal pain score; AS: ankylosing spondylitis; bDMARD-IR: inadequate response or intolerant to biologic disease-modifying antirheumatic drug; CD: Crohn’s disease; CDAI: Crohn’s Disease Activity Index; CRP: C-reactive protein; JAK: Janus kinase; nr-axSpA: non-radiographic axial spondyloarthritis; PsA: psoriatic arthritis; QD: once daily; RA: rheumatoid arthritis; SES-CD: simple endoscopic activity score for Crohn’s disease; SF: stool frequency; UC: ulcerative colitis.
Study designs: the U-EXCEL and U-EXCEED induction studies were both multicenter, double-blind, placebo-controlled clinical studies. In U-EXCEL (N=526 [287 bio-naive, 239 biologic failures]) and U-EXCEED (N=495 biologic failures only), patients were randomized to RINVOQ 45 mg QD or placebo for 12 weeks with a 2:1 treatment allocation ratio and included in the efficacy analysis. In both studies, induction nonresponders were allowed to enter an additional 12-week open-label extended treatment period. All enrolled patients had moderately to severely active CD defined as SF ≥4 and/or APS ≥2, plus an SES-CD ≥6 (≥4 for patients with isolated ileal disease) excluding the narrowing component. U-ENDURE maintenance was a multicenter, double-blind, placebo-controlled clinical study with 502 patients who achieved clinical response (≥30% decrease in average daily SF and/or in APS, neither worse than baseline) to 12 weeks of RINVOQ 45 mg QD induction treatment. These patients were rerandomized 1:1:1 to receive either RINVOQ 15 mg QD, 30 mg QD, or placebo.1
UP NEXT
RINVOQ is an oral, once daily, selective and reversible JAK inhibitor now approved for the treatment of adult patients with moderately to severely active Crohn’s disease who have had an inadequate response, lost response or were intolerant to either conventional therapy or a biologic agent.1
RINVOQ can be taken at any time of the day, with or without food. Tablets should be swallowed whole and should not be split, crushed, or chewed in order to ensure the entire dose is delivered correctly.1
A Phase 3 clinical trial program involving 3 studies: 2 12-week induction studies evaluated RINVOQ 45 mg QD vs placebo, and 1 52-week maintenance treatment and long-term extension study evaluated RINVOQ 15 mg QD and RINVOQ 30 mg QD vs placebo.1
Learn more about RINVOQ in our quick introductory video.
[Affiliates to insert local summary of safety]
REFERENCES
- RINVOQ [Summary of Product Characteristics]. AbbVie Deutschland GmbH & Co. KG; April 2023.
- Burmester GR, Kremer JM, Van den Bosch F, et al. Safety and efficacy of upadacitinib in patients with rheumatoid arthritis and inadequate response to conventional synthetic disease-modifying anti-rheumatic drugs (SELECT-NEXT): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2018;391(10139):2503-2512. doi:10.1016/S0140-6736(18)31115-2
- Data on file, AbbVie Inc. ABVRRTI74825.
- van Vollenhoven R, Takeuchi T, Pangan AL, et al. Efficacy and safety of upadacitinib monotherapy in methotrexate-naive patients with moderately-to-severely active rheumatoid arthritis (SELECT-EARLY): a multicenter, multi-country, randomized, double-blind, active comparator-controlled trial. Arthritis Rheumatol. 2020;72(10):1607-1620. doi:10.1002/art.41384
- Fleischmann R, Pangan AL, Song IH, et al. Upadacitinib versus placebo or adalimumab in patients with rheumatoid arthritis and an inadequate response to methotrexate: results of a phase III, double-blind, randomized controlled trial. Arthritis Rheumatol. 2019;71(11):1788-1800. doi:10.1002/art.41032
- Smolen JS, Pangan AL, Emery P, et al. Upadacitinib as monotherapy in patients with active rheumatoid arthritis and inadequate response to methotrexate (SELECT-MONOTHERAPY): a randomised, placebo-controlled, double-blind phase 3 study. Lancet. 2019;393(10188):2303-2311. doi:10.1016/S0140-6736(19)30419-2
- Genovese MC, Fleischmann R, Combe B, et al. Safety and efficacy of upadacitinib in patients with active rheumatoid arthritis refractory to biologic disease-modifying anti-rheumatic drugs (SELECT-BEYOND): a double-blind, randomised controlled phase 3 trial. Lancet. 2018;391(10139):2513-2524. doi:10.1016/S0140-6736(18)31116-4
- Rubbert-Roth A, Enejosa J, Pangan AL, et al. Trial of upadacitinib or abatacept in rheumatoid arthritis. N Engl J Med. 2020;383(16):1511-1521. doi:10.1056/NEJMoa2008250
- McInnes IB, Anderson JK, Magrey M, et al. Trial of upadacitinib and adalimumab for psoriatic arthritis. N Engl J Med. 2021;384(13):1227-1239. doi:10.1056/NEJMoa2022516
- Mease PJ, Lertratanakul A, Anderson JK, et al. Upadacitinib for psoriatic arthritis refractory to biologics: SELECT-PsA 2. Ann Rheum Dis. 2021;80(3):312-320. doi:10.1136/annrheumdis-2020-218870
- Deodhar A, Van den Bosch F, Poddubnyy D, et al. Upadacitinib for the treatment of active non-radiographic axial spondyloarthritis (SELECT-AXIS 2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2022;400(10349):369-379. doi:10.1016/S0140-6736(22)01212-0
- van der Heijde D, Song IH, Pangan AL, et al. Efficacy and safety of upadacitinib in patients with active ankylosing spondylitis (SELECT-AXIS 1): a multicentre, randomised, double-blind, placebo-controlled, phase 2/3 trial. Lancet. 2019;394(10214):2108-2117. doi:10.1016/S0140-6736(19)32534-6
- van der Heijde D, Baraliakos X, Sieper J, et al. Efficacy and safety of upadacitinib for active ankylosing spondylitis refractory to biological therapy: a double-blind, randomised, placebo-controlled phase 3 trial. Ann Rheum Dis. 2022;81(11):1515-1523. doi:10.1136/ard-2022-222608
- Guttman-Yassky E, Teixeira HD, Simpson EL, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021;397(10290):2151-2168. doi:10.1016/S0140-6736(21)00588-2
- Reich K, Teixeira HD, de Bruin-Weller M, et al. Safety and efficacy of upadacitinib in combination with topical corticosteroids in adolescents and adults with moderate-to-severe atopic dermatitis (AD Up): results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2021;397(10290):2169-2181. doi:10.1016/S0140-6736(21)00589-4
- Blauvelt A, Teixeira HD, Simpson EL, et al. Efficacy and safety of upadacitinib vs dupilumab in adults with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2021;157(9):1047-1055. doi:10.1001/jamadermatol.2021.3023
- Danese S, Vermeire S, Zhou W, et al. Upadacitinib as induction and maintenance therapy for moderately to severely active ulcerative colitis: results from three phase 3, multicentre, double-blind, randomised trials. Lancet. 2022;399(10341):2113-2128. doi:10.1016/S0140-6736(22)00581-5
- RINVOQ [package insert]. North Chicago, IL: AbbVie Inc.
- European Commission approves AbbVie's RINVOQ™ (upadacitinib) for the treatment of psoriatic arthritis and ankylosing spondylitis. News release. AbbVie. January 25, 2021. Accessed February 24, 2023. https://news.abbvie.com/alert-topics/immunology/european-commission-approves-abbvies-rinvoq-upadacitinib-for-treatment-psoriatic-arthritis-and-ankylosing-spondylitis.htm
- RINVOQ® (upadacitinib) receives its sixth U.S. FDA approval. News release. AbbVie. October 21, 2022. Accessed February 24, 2023. https://news.abbvie.com/news/press-releases/rinvoq-upadacitinib-receives-its-sixth-us-fda-approval.htm
- RINVOQ® (upadacitinib) approved by European Commission as an oral treatment for adults with active non-radiographic axial spondyloarthritis. News release. AbbVie. July 29, 2022. Accessed February 24, 2023. https://news.abbvie.com/news/press-releases/rinvoq-upadacitinib-approved-by-european-commission-as-an-oral-treatment-for-adults-with-active-non-radiographic-axial-spondyloarthritis.htm
- European Commission approves RINVOQ® (upadacitinib) for the treatment of adults with moderate to severe ulcerative colitis. News release. AbbVie. July 26, 2022. Accessed February 24, 2023. https://news.abbvie.com/alert-topics/immunology/european-commission-approves-rinvoq-upadacitinib-for-treatment-adults-with-moderate-to-severe-ulcerative-colitis.htm
- AbbVie announces European Commission approval of RINVOQ® (upadacitinib) for the treatment of moderately to severely active Crohn's disease. News release. AbbVie. April 17, 2023. Accessed April 17, 2023. https://news.abbvie.com/news/press-releases/abbvie-anno. Aprunces-european-commission-approval-rinvoq-upadacitinib-for-treatment-moderately-to-severely-active-crohns-disease.htm
- European Commission approves RINVOQ® (upadacitinib) as first JAK inhibitor in the European Union for the treatment of both adults and adolescents with moderate to severe atopic dermatitis. News release. AbbVie. August 24, 2021. Accessed February 24, 2023. https://news.abbvie.com/news/press-releases/european-commission-approves-rinvoq-upadacitinib-as-first-jak-inhibitor-in-european-union-for-treatment-both-adults-and-adolescents-with-moderate-to-severe-atopic-dermatitis.htm