DRAFT FOR INTERNAL USE ONLY
This DRAFT of the Global RINVOQ CD AbbVie Pro (v0.2) has been developed based on the DRAFT RINVOQ CD CLM, ALL-RNQG-220127 (v1.0).
RINVOQ CD was approved by the European Commission in April 2023 and the DRAFT CLM is being updated accordingly but is not final approved. When implemented locally, affiliates must align AbbVie Pro to the forthcoming final approved RINVOQ CD CLM, ALL-RNQG-220127 (version to be determined).
Note to Affiliates: Please evaluate use of ALL claims, graphs/tables, and corresponding references (e.g., data on file, abstracts, posters, manuscripts) according to local standards, codes, and regulations.
RINVOQ® (upadacitinib) is indicated for the treatment of adult patients with moderately to severely active Crohnʼs disease (CD) who have had an inadequate response, lost response or were intolerant to either conventional therapy or a biologic agent.1
RINVOQ achieved its co-primary endpoints of endoscopic response and clinical remission at Week 12 (Induction) and Week 52 (Maintenance)1
▼ This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions.
A Phase 3 trial program involving 3 studies:1
2 induction studies (U-EXCEL and U-EXCEED) and 1 maintenance study (U-ENDURE). 1,021 moderately to severely active CD patients evaluating RINVOQ 45 mg QD vs placebo for induction, and RINVOQ 15 mg QD or 30 mg QD vs placebo for maintenance treatment (N=502).1*
*Patients who achieved clinical response (≥30% decrease in average daily stool frequency and/or in abdominal pain score, neither worse than baseline) to 12 weeks of RINVOQ 45 mg QD induction treatment entered maintenance.
ONE PILL, ONCE A DAY1
RINVOQ is a once-daily pill that comes in 3 dosage strengths1
Note to Affiliates: Please verify tablet images for accuracy and reference based on local labeling.
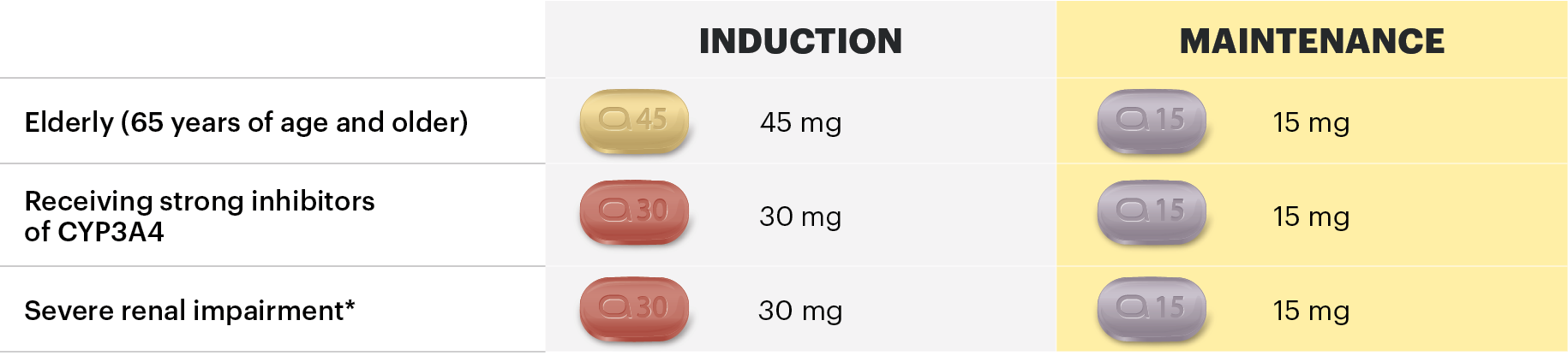
The recommended starting dose of RINVOQ is 45 mg QD for 12 weeks.
For patients who have not achieved adequate therapeutic benefit after the initial 12-week induction, prolonged induction for additional 12 weeks with a dose of 30 mg once daily may be considered. For these patients, RINVOQ should be discontinued if there is no evidence of therapeutic benefit after 24 weeks of treatment.
The recommended maintenance dose of RINVOQ is 15 mg or 30 mg QD based on individual patient presentation:
A dose of 30 mg QD may be appropriate for patients with high disease burden who are not at higher risk of VTE, MACE, and malignancy or who do not show adequate therapeutic benefit with 15 mg QD
For patients 65 years of age and older, the recommended maintenance dose is 15 mg QD.
In patients who have responded to treatment with RINVOQ, corticosteroids may be reduced and/or discontinued in accordance with standard of care.
Pill size not to scale (14mm)
RINVOQ is a small molecule without the concern of immunogenicity2
DOSING IN SPECIAL PATENT POPULATIONS1
Dose initiation1
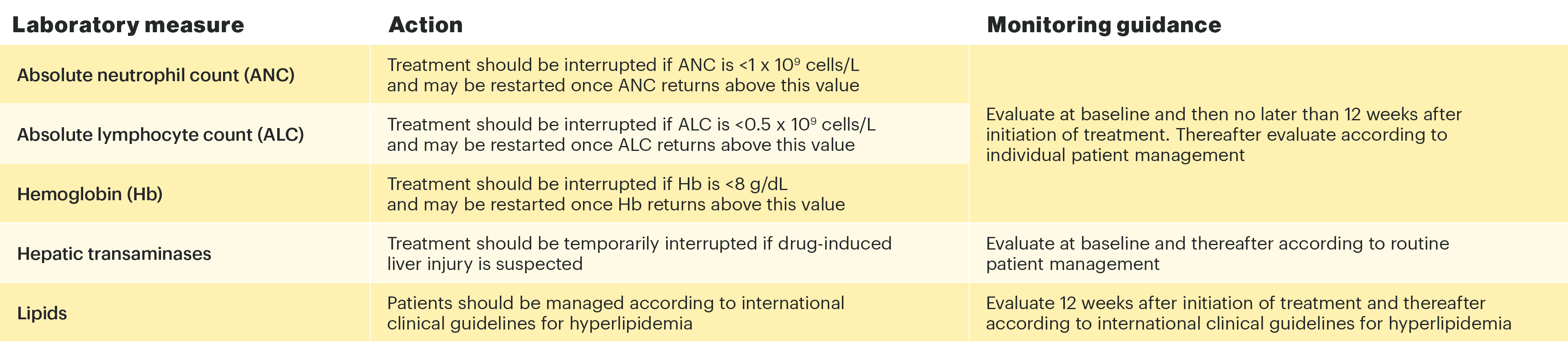
Treatment should not be initiated in patients with an absolute lymphocyte count that is <0.5 x 109 cells/L, an absolute neutrophil count that is <1 x 109 cells/L, or who have hemoglobin levels that are <8 g/dL.
Dose interruption1
Treatment should be interrupted if a patient develops a serious infection until the infection is controlled. Interruption of dosing may be needed for management of laboratory abnormalities.
Interactions and coadministration1
For patients with Crohn’s disease receiving strong inhibitors of CYP3A4 (e.g., ketoconazole, clarithromycin), the recommended induction dose is 30 mg QD and the recommended maintenance dose is 15 mg QD.
Combination with other potent immunosuppressants such as azathioprine, 6-mercaptopurine, ciclosporin, tacrolimus, and biologic DMARDs or other JAK inhibitors has not been evaluated in clinical studies and is not recommended as a risk of additive immunosuppression cannot be excluded.
In patients who have responded to induction or maintenance treatment with RINVOQ, steroids may be reduced and/or discontinued in accordance with standard of care.
Laboratory measures and monitoring guidance1
Special warnings and precautions for use
Gastrointestinal perforations: RINVOQ should be used with caution in patients who may be at risk for gastrointestinal perforation (e.g., patients with diverticular disease, a history of diverticulitis, or who are taking NSAIDs, corticosteroids, or opioids). Patients presenting with new onset abdominal signs and symptoms should be evaluated promptly for early identification of diverticulitis or gastrointestinal perforation.
Serious infections: RINVOQ should not be initiated in patients with an active, serious infection, including localised infections. Consider the risks and benefits of treatment prior to initiating RINVOQ in patients: with chronic or recurrent infection; who have been exposed to tuberculosis; with a history of a serious or an opportunistic infection; who have resided or traveled in areas of endemic tuberculosis or endemic mycoses; or with underlying conditions that may predispose them to infection. Patients should be closely monitored for the development of signs and symptoms of infection during and after treatment with RINVOQ. RINVOQ therapy should be interrupted if a patient develops a serious or opportunistic infection. A patient who develops a new infection during treatment with RINVOQ should undergo prompt and complete diagnostic testing appropriate for an immunocompromised patient; appropriate antimicrobial therapy should be initiated, the patient should be closely monitored, and RINVOQ therapy should be interrupted if the patient is not responding to antimicrobial therapy. RINVOQ therapy may be resumed once the infection is controlled.
Tuberculosis: Patients should be screened for TB before starting RINVOQ therapy. RINVOQ should not be given to patients with active TB. Anti-TB therapy should be considered prior to initiation of RINVOQ in patients with previously untreated latent TB or in patients with risk factors for TB infection. Patients should be monitored for the development of signs and symptoms of TB, including patients who tested negative for latent TB infection prior to initiating therapy.
Viral reactivation: If a patient develops herpes zoster, interruption of RINVOQ therapy should be considered until the episode resolves. Screening for viral hepatitis and monitoring for reactivation should be performed before starting and during therapy with RINVOQ. If hepatitis B virus DNA is detected while receiving RINVOQ, a liver specialist should be consulted.
Venous thromboembolism: In patients with known VTE risk factors other than cardiovascular or malignancy risk factors, RINVOQ should be used with caution. VTE risk factors other than cardiovascular or malignancy risk factors include previous VTE, patients undergoing major surgery, immobilization, use of combined hormonal contraceptives or hormone replacement therapy, and inherited coagulation disorder. Patients should be re-evaluated periodically during RINVOQ treatment to assess for changes in VTE risk. Promptly evaluate patients with signs and symptoms of VTE and discontinue RINVOQ in patients with suspected VTE, regardless of dose.
Nonmelanoma skin cancer: Periodic skin examination is recommended for patients who are at increased risk for skin cancer.
APS: abdominal pain score; CYP3A4: cytochrome P450 3A4; DMARD: disease-modifying antirheumatic drug; JAK: Janus kinase; MACE: major adverse cardiac event; NSAID: nonsteroidal anti-inflammatory drug; QD: once daily; SES-CD: simple endoscopic activity score for Crohn’s disease; SF: stool frequency; TB: tuberculosis; VTE: venous thromboembolism.
Study designs: the U-EXCEL and U-EXCEED induction studies were both multicenter, double-blind, placebo-controlled clinical studies. In U-EXCEL (N=526 [287 bio-naive, 239 biologic failures]) and U-EXCEED (N=495 biologic failures only), patients were randomized to RINVOQ 45 mg QD or placebo for 12 weeks with a 2:1 treatment allocation ratio and included in the efficacy analysis. In both studies, induction nonresponders were allowed to enter an additional 12-week open-label extended treatment period. All enrolled patients had moderately to severely active CD defined as SF ≥4 and/or APS ≥2, plus an SES-CD ≥6 (≥4 for patients with isolated ileal disease) excluding the narrowing component. U-ENDURE maintenance was a multicenter, double-blind, placebo-controlled clinical study with 502 patients who achieved clinical response (≥30% decrease in average daily SF and/or in APS, neither worse than baseline) to 12 weeks of RINVOQ 45 mg QD induction treatment. These patients were rerandomized 1:1:1 to receive either RINVOQ 15 mg QD, 30 mg QD, or placebo.1
UP NEXT
RINVOQ is an oral, once daily, selective and reversible JAK inhibitor now approved for the treatment of adult patients with moderately to severely active Crohn’s disease who have had an inadequate response, lost response or were intolerant to either conventional therapy or a biologic agent.1
RINVOQ can be taken at any time of the day, with or without food. Tablets should be swallowed whole and should not be split, crushed, or chewed in order to ensure the entire dose is delivered correctly.1
A Phase 3 clinical trial program involving 3 studies: 2 12-week induction studies evaluated RINVOQ 45 mg QD vs placebo, and 1 52-week maintenance treatment and long-term extension study evaluated RINVOQ 15 mg QD and RINVOQ 30 mg QD vs placebo.1
Learn more about RINVOQ in our quick introductory video.
[Affiliates to insert local summary of safety]
REFERENCES
- RINVOQ [Summary of Product Characteristics]. AbbVie Deutschland GmbH & Co. KG; April 2023.
- Pérez-Jeldres T, Tyler CJ, Boyer JD, et al. Targeting cytokine signaling and lymphocyte traffic via small molecules in inflammatory bowel disease: JAK inhibitors and S1PR agonists. Front Pharmacol. 2019;10:212. doi:10.3389/fphar.2019.00212