DRAFT FOR INTERNAL USE ONLY
This DRAFT of the Global RINVOQ CD AbbVie Pro (v0.2) has been developed based on the DRAFT RINVOQ CD CLM, ALL-RNQG-220127 (v1.0).
RINVOQ CD was approved by the European Commission in April 2023 and the DRAFT CLM is being updated accordingly but is not final approved. When implemented locally, affiliates must align AbbVie Pro to the forthcoming final approved RINVOQ CD CLM, ALL-RNQG-220127 (version to be determined).
Note to Affiliates: Please evaluate use of ALL claims, graphs/tables, and corresponding references (e.g., data on file, abstracts, posters, manuscripts) according to local standards, codes, and regulations.
RINVOQ® (upadacitinib) is indicated for the treatment of adult patients with moderately to severely active Crohnʼs disease (CD) who have had an inadequate response, lost response or were intolerant to either conventional therapy or a biologic agent.1
RINVOQ achieved its co-primary endpoints of endoscopic response and clinical remission at Week 12 (Induction) and Week 52 (Maintenance)1
▼ This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions.
[Note to Affiliates: HCP-delivered patient materials and their inclusion herein are subject to local codes and regulations.]
RINVOQ is an oral, once daily, selective and reversible JAK inhibitor now approved for the treatment of adult patients with moderately to severely active Crohn’s disease who have had an inadequate response, lost response or were intolerant to either conventional therapy or a biologic agent.1
RINVOQ can be taken at any time of the day, with or without food. Tablets should be swallowed whole and should not be split, crushed, or chewed in order to ensure the entire dose is delivered correctly.1
A Phase 3 clinical trial program involving 3 studies: 2 12-week induction studies evaluated RINVOQ 45 mg QD vs placebo, and 1 52-week maintenance treatment and long-term extension study evaluated RINVOQ 15 mg QD and RINVOQ 30 mg QD vs placebo.1
Learn more about RINVOQ in our quick introductory video.
[ NEW VIDEOS TO COME ]
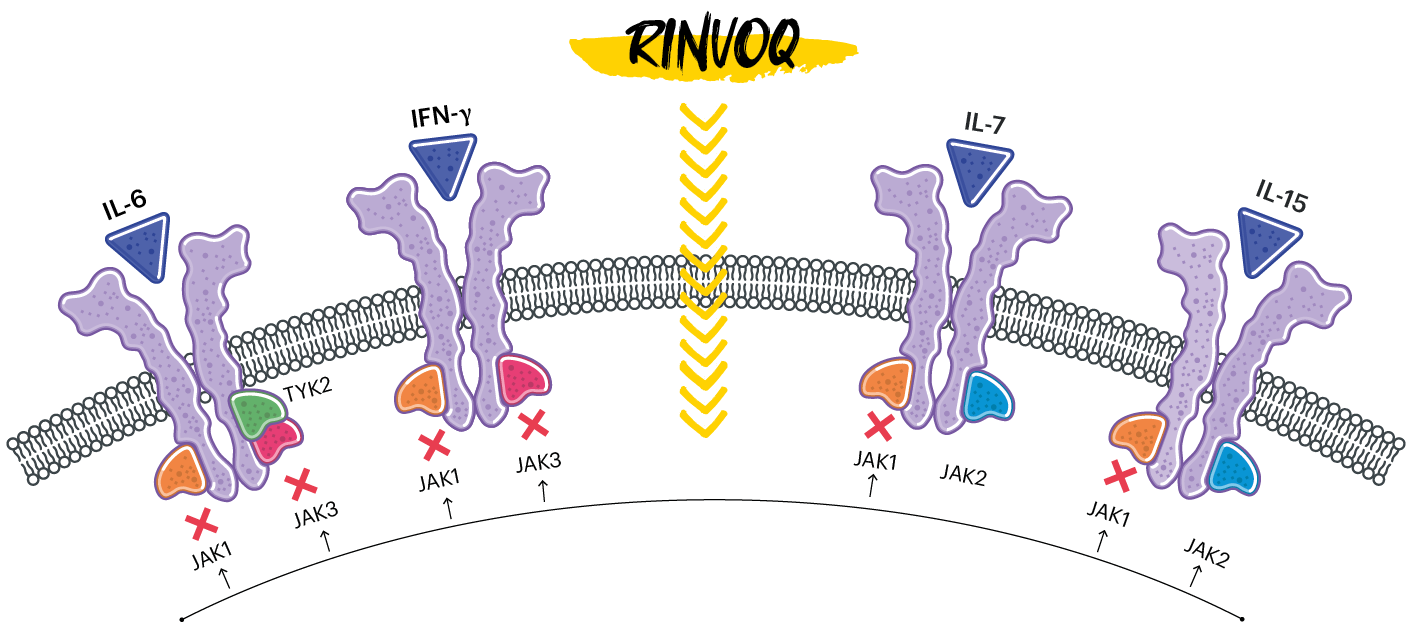
RINVOQ IS A SELECTIVE AND REVERSIBLE JAK INHIBITOR1
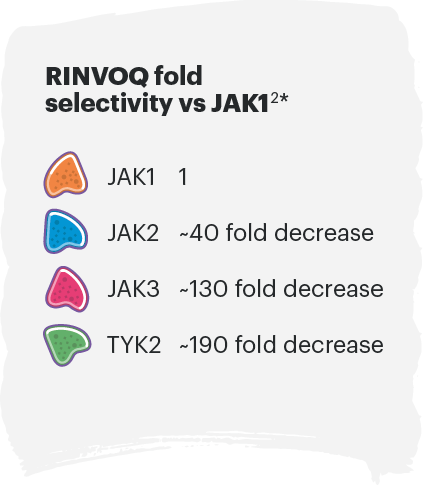
In human cellular assays, RINVOQ preferentially inhibits signaling by JAK1 or JAK1/3 with functional selectivity over cytokine receptors that signal via pairs of JAK2.1
(RINVOQ's JAK inhibition is represented by X)
*In vitro potency in engineered cellular assays. Fold selectivity is indicative of how much more selective RINVOQ is to JAK1 over other JAKs.
[Affiliate to create and gain local MLOR approval of this PDF, including ISI]
APS: abdominal pain score; JAK: Janus kinase; IL: interleukin; SES-CD: simple endoscopic activity score for Crohn’s disease; SF: stool frequency.
Study designs: the U-EXCEL and U-EXCEED induction studies were both multicenter, double-blind, placebo-controlled clinical studies. In U-EXCEL (N=526 [287 bio-naive, 239 biologic failures]) and U-EXCEED (N=495 biologic failures only), patients were randomized to RINVOQ 45 mg Q or placebo for 12 weeks with a 2:1 treatment allocation ratio and included in the efficacy analysis. In both studies, induction nonresponders were allowed to enter an additional 12-week open-label extended treatment period. All enrolled patients had moderately to severely active CD defined as SF ≥4 and/or APS ≥2, plus an SES-CD ≥6 (≥4 for patients with isolated ileal disease) excluding the narrowing component. U-ENDURE maintenance was a multicenter, double-blind, placebo-controlled clinical study with 502 patients who achieved clinical response (≥30% decrease in average daily SF and/or in APS, neither worse than baseline) to 12 weeks of RINVOQ 45 mg QD induction treatment. These patients were rerandomized 1:1:1 to receive either RINVOQ 15 mg QD, 30 mg QD, or placebo.1
LOOKING FOR MORE INFORMATION?
[Affiliates to insert local summary of safety]
REFERENCES
- RINVOQ [Summary of Product Characteristics]. AbbVie Deutschland GmbH & Co. KG; April 2023.
- Parmentier JM, Voss J, Graff C, et al. In vitro and in vivo characterization of the JAK1 selectivity of upadacitinib (ABT-494). BMC Rheumatol. 2018;2:23. doi:10.1186/s41927-018-0031-x