RINVOQ® (upadacitinib) is indicated for the treatment of adult patients with moderately to severely active ulcerative colitis (UC) who have had an inadequate response, lost response or were intolerant to either conventional therapy or a biologic agent.1
RINVOQ achieved the primary endpoints of clinical remission per adapted Mayo score at Induction Week 8 and Maintenance Week 521,2
▼ This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions.
A Phase 3 trial program involving 3 studies: 2 replicate induction studies (U-ACHIEVE Induction and U-ACCOMPLISH) and 1 maintenance study (U-ACHIEVE Maintenance). A total of 988 patients with moderately to severely active UC evaluating RINVOQ 45 mg QD vs placebo for induction and RINVOQ 15 mg QD and 30 mg QD vs placebo for maintenance treatment (N=451).1*
*Patients who achieved clinical response per adapted Mayo score with 8-week RINVOQ 45 mg QD induction treatment entered maintenance.
*p<0.001 vs placebo, multiplicity-controlled analysis (ITT)
The primary endpoints of both studies was achievement of remission per adapted Mayo score at Week 8.1,2
Study design: U-ACHIEVE Induction (UC-1) and U-ACCOMPLISH (UC-2) were replicate induction studies, both of which were multicenter, double-blind, placebo-controlled clinical studies. In UC-1 and UC-2, 988 patients (473 and 515 patients, respectively) were randomized to RINVOQ 45 mg QD or placebo for 8 weeks with a 2:1 treatment allocation ratio and included in the efficacy analysis. All enrolled patients had moderately to severely active UC defined as aMs of 5 to 9 with an ESS of 2 or 3 and demonstrated prior treatment failure including inadequate response, loss of response, or intolerance to prior conventional and/or biologic treatment.1,2
*p<0.001 vs placebo, multiplicity-controlled analysis (ITT)
Clinical response per partial adapted Mayo, also known as symptomatic response, is defined as a decrease in partial adapted Mayo score ≥1 point and ≥30% from baseline, PLUS a decrease in rectal bleeding score ≥1 or an absolute rectal bleeding score ≤1.1,2 Error bars show 95% CI. Clinical response per paMs at Week 2 was a ranked secondary endpoint in both induction (U-ACHIEVE and U-ACCOMPLISH).1,2
Study design: U-ACHIEVE Induction (UC-1) and U-ACCOMPLISH (UC-2) were replicate induction studies, both of which were multicenter, double-blind, placebo-controlled clinical studies. In UC-1 and UC-2, 988 patients (473 and 515 patients, respectively) were randomized to RINVOQ 45 mg QD or placebo for 8 weeks with a 2:1 treatment allocation ratio and included in the efficacy analysis. All enrolled patients had moderately to severely active UC defined as aMs of 5 to 9 with an ESS of 2 or 3 and demonstrated prior treatment failure including inadequate response, loss of response, or intolerance to prior conventional and/or biologic treatment.1,2
Post-hoc analysis of U‑ACHIEVE and U‑ACCOMPLISH (pooled)
POST-HOC LIMITATIONS Post-hoc analyses are analyses of data taken from already completed trials, and all endpoints analysed in a post-hoc analysis were not prespecified in the original trial.4
Key challenges of post-hoc analyses include lack of multiplicity control, having no prespecified endpoints before the clinical trial has begun, as well as statistical versus clinical significance.4-6
Because of this, post-hoc subgroup analyses can only be used to generate hypotheses, rather than making strong inferences.4
*Nominal p<0.001 vs placebo. †Nominal p<0.05 vs placebo; not multiplicity controlled. Error bars show 95% CI. Day 0 represents the first day of randomization and the first day of treatment. No clinical inferences can be drawn from this data.
Patients were instructed to use an electronic diary to record daily symptoms during the studies, including stool frequency and rectal bleeding. Mayo stool frequency was determined by patients recording the normal number of bowel movements when their UC was not active in a 24-hour period (i.e. a score of 0 = normal number of stools for subject). Scoring for rectal bleeding was as follows: 0, no blood seen; 1, streaks of blood with stool less than half the time; 2, obvious blood with stool most of the time; 3, blood alone passed.3
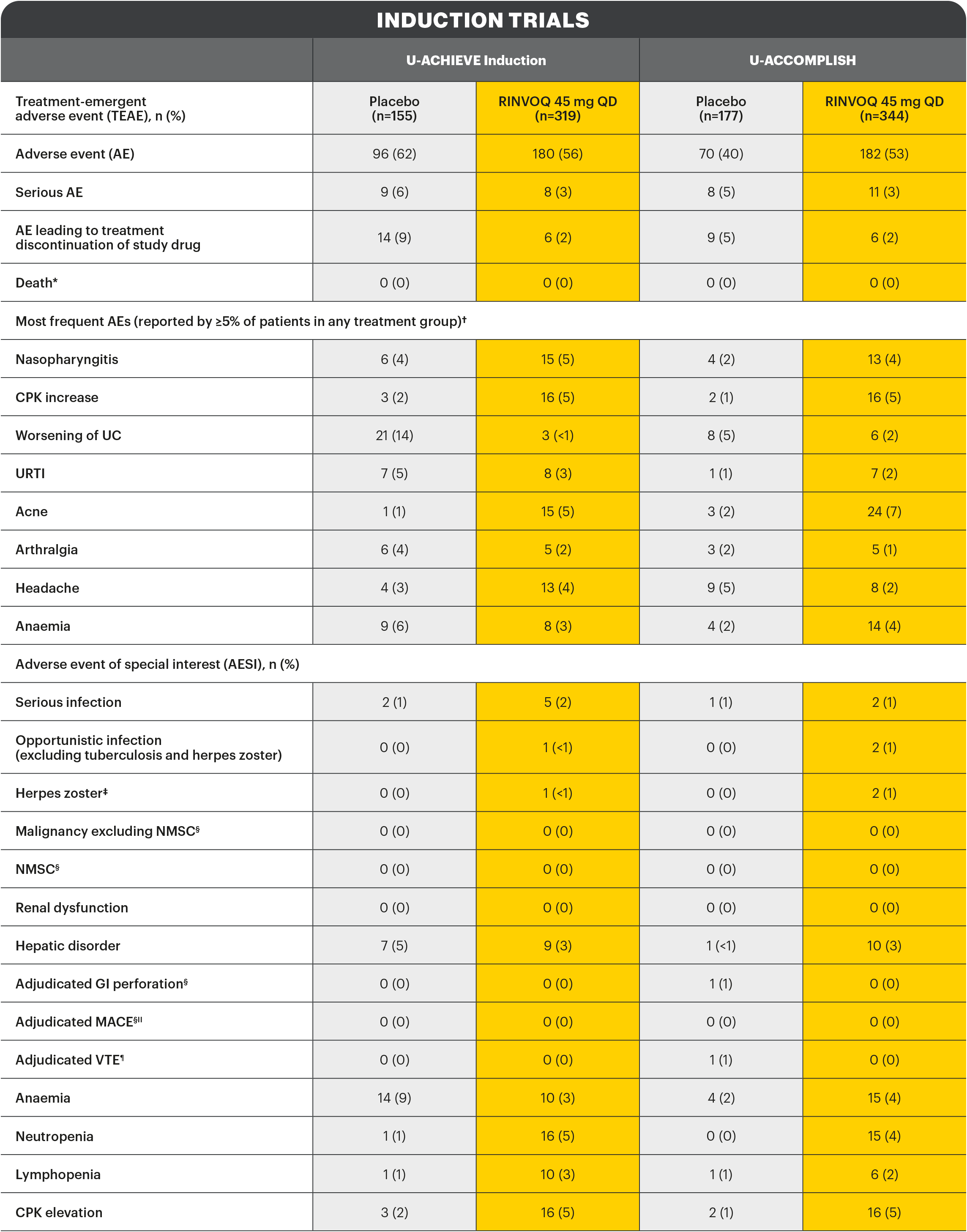
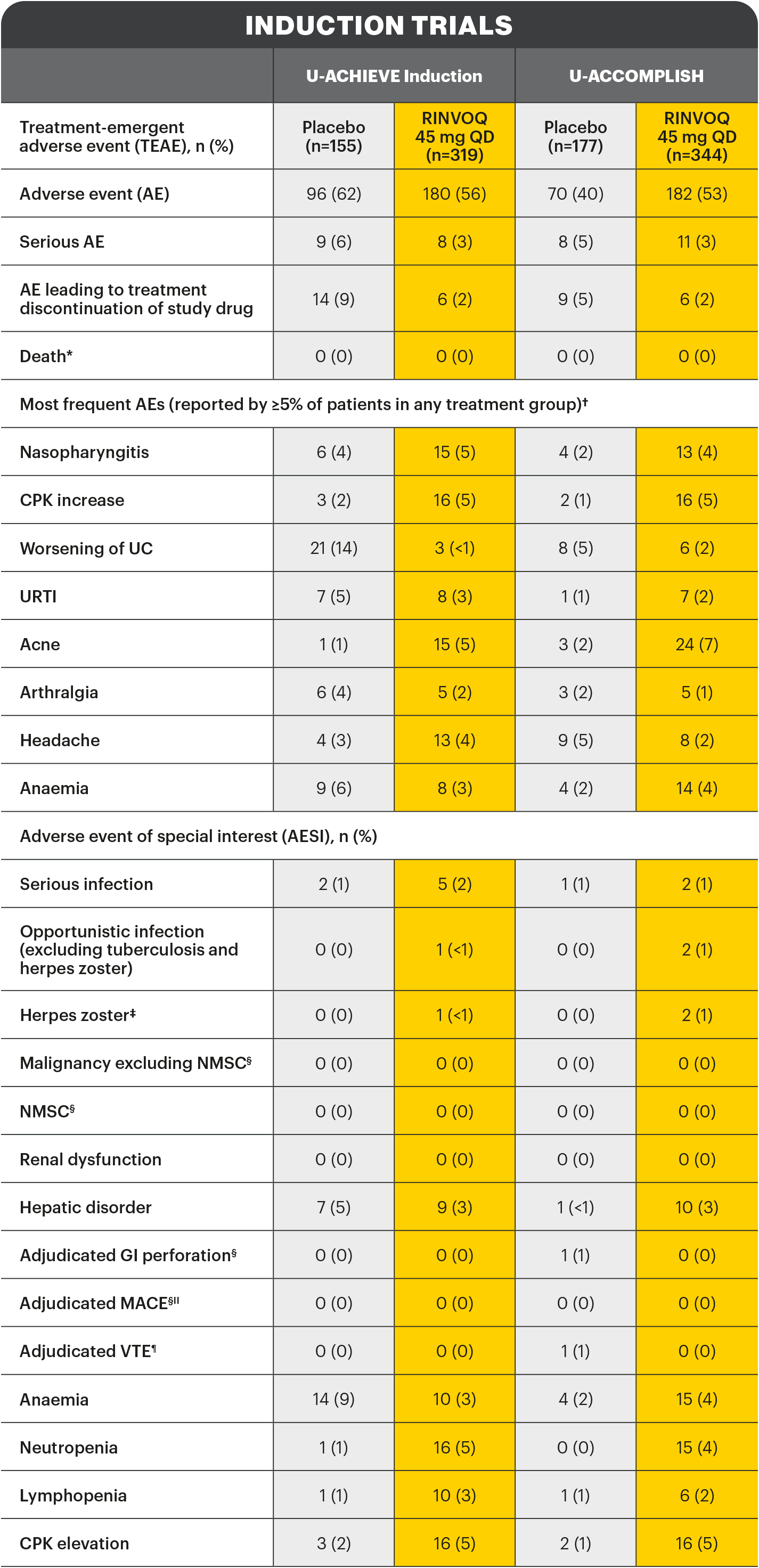
*Includes non-treatment emergent deaths. †The rates of the four most frequent adverse events are listed for all studies. ‡Search criteria were based on Company MedDRA Query. §These events were determined on the basis of external adjudication. IIMACE is defined as cardiovascular death, non-fatal myocardial infarction, and non-fatal stroke. ¶VTE is defined as deep vein thrombosis ad pulmonary embolism (fatal and non-fatal).
There were no AESIs of active tuberculosis or lymphoma in the study.2
AE: adverse event; AESI: adverse event of special interest; aMs: adapted Mayo score; CI: confidence interval; CPK: creatine phosphokinase; ESS: endoscopic subscore; GI: gastrointestinal; ITT: intention to treat; MACE: major adverse cardiac event; NMSC: non-melanoma skin cancer; NRI-C: non-responder imputation incorporating multiple imputations to handle missing data due to coronavirus disease 2019 (COVID-19); paMs: partial adapted Mayo score; QD: once-daily; RBS: rectal bleeding score; TEAE: treatment-emergent adverse event; UC: ulcerative colitis; URTI: upper respiratory tract infection; VTE: venous thromboembolism.
RINVOQ is an oral, once daily, selective and reversible JAK inhibitor now approved for the treatment of adult patients with moderately to severely active ulcerative colitis who have had an inadequate response, lost response or were intolerant to either conventional therapy or a biologic agent.1
RINVOQ can be taken at any time of the day, with or without food.1
A Phase 3 clinical trial program involving 3 studies: 2 replicate induction studies and 1 maintenance study evaluated RINVOQ 45 mg vs placebo for induction, and RINVOQ 15 mg and 30 mg vs placebo for maintenance treatment.
[Please insert local summary of safety]
REFERENCES
- RINVOQ Summary of Product Characteristics [DRAFT].
- Danese S, Vermeire S, Zhou W, et al. Lancet. Published online May 26, 2022. doi: https://doi.org/10.1016/S0140-6736(22)00581-5.
- Vermeire S, Colombel JF, Takeuchi K, et al. Journal of Crohn's and Colitis. 2022; 16 (Supplement_1), i087–i088. doi: https://doi.org/10.1093/ecco-jcc/jjab232.077.
- Srinivas TR, Bing H, et al. Transplantation. 2015;5(99):17-20.
- Dmitrienko A and D'Agostino RBD Sr. Stat Med. 2017;36(28):4423–4426.
- Ranganathan R, Pramesh CS and Buyse M. Perspect Clin Res. 2015;6:169-170.