SKYRIZI (risankizumab) is an IL-23/p19 inhibitor indicated for the treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic therapy. SKYRIZI, alone or in combination with MTX, is also indicated for the treatment of active psoriatic arthritis in adults who have had an inadequate response or who have been intolerant to one or more DMARDs.1
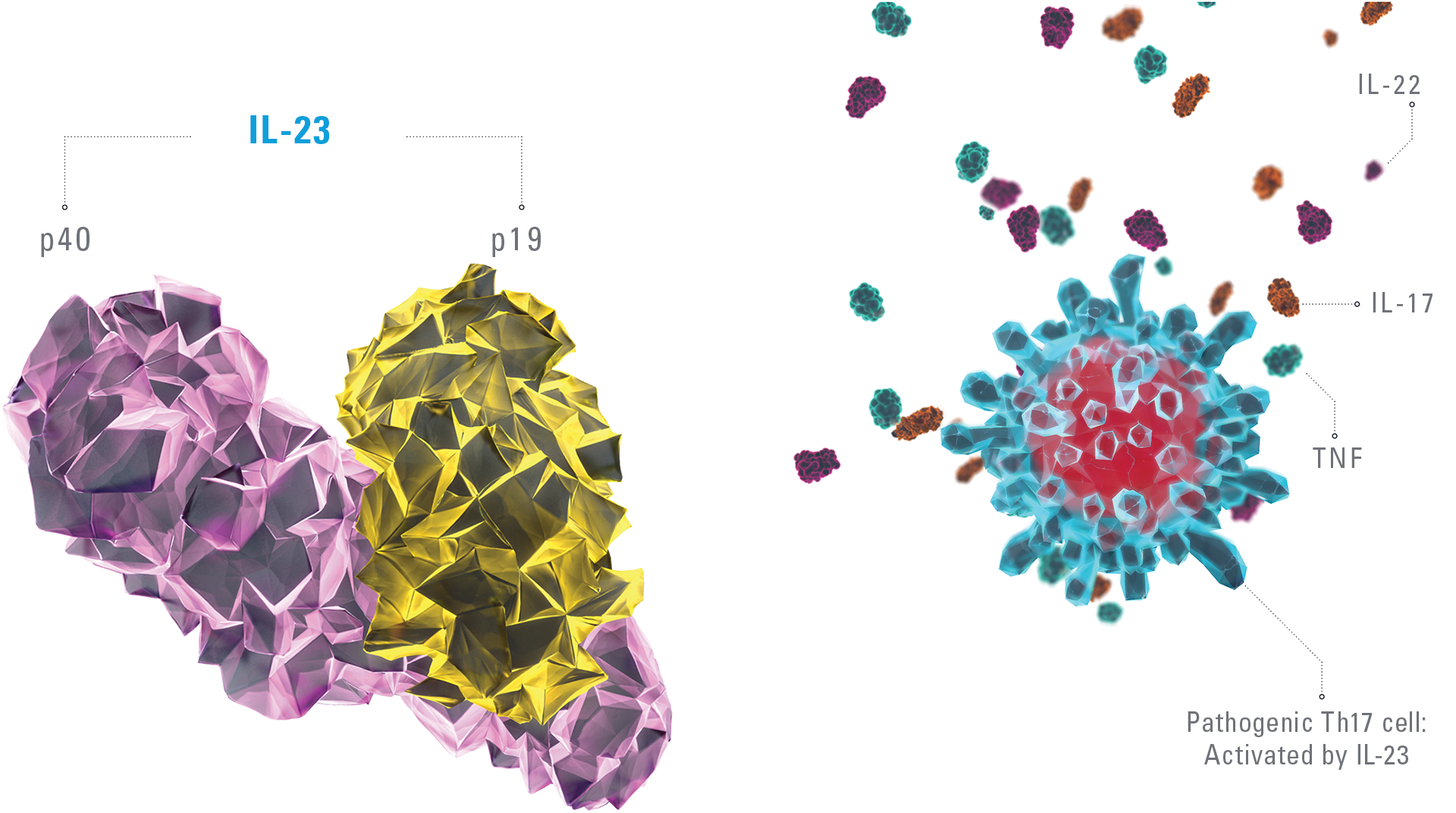
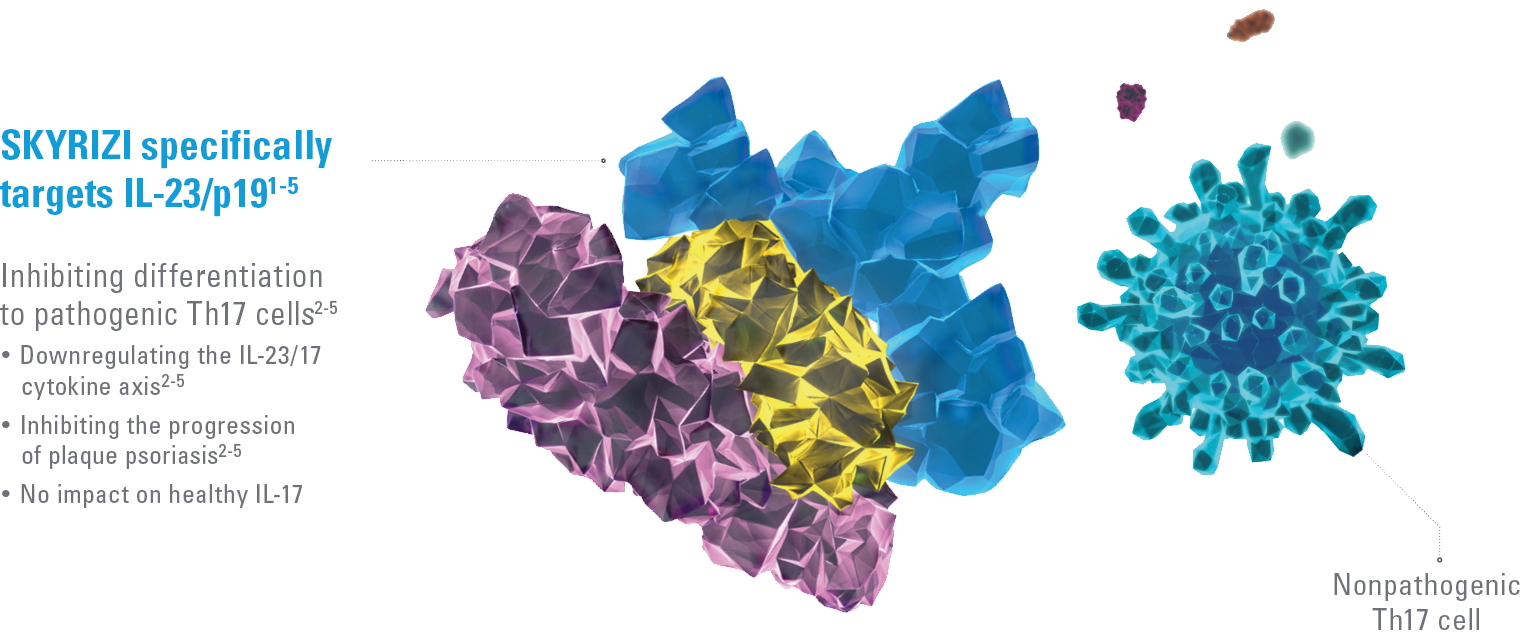
SKYRIZI BINDS WITH HIGH AFFINITY AND SPECIFICITY TO IL-23/P191-5
The IL-23/IL-17 cytokine axis is central to the psoriatic disease process.2-8 By binding to the p19 subunit of IL-23, SKYRIZI downregulates the IL-23/17 cytokine axis that drives the pathogenicity of psoriasis.2-5 Targeting IL-23 has no impact on the nonpathogenic Th17 pathway.3,8-11
ACR=American College of Rheumatology; ADA=adalimumab; AE=adverse events; bio-IR=inadequate response to a biologic; BMI=body mass index; CI=confidence interval; csDMARD-IR=inadequate response to a conventional synthetic DMARD; DLQI=Dermatology Life Quality Index; DMARD=disease-modifying antirheumatic drug; E=event; IBD=inflammatory bowel disease; IL=interleukin; LDI=Leeds Dactylitis Index; LEI=Leeds Enthesitis Index; MACE=major adverse cardiovascular event; MTX=methotrexate; NMSC=nonmelanoma skin cancer; NRI=nonresponder imputation; OC=observed case; OLE=open-label extension; PASI=Psoriasis Area and Severity Index; PBO=placebo; PsA=psoriatic arthritis; PsO=psoriasis; PSS=psoriasis symptom scale; PY=patient-year; RCT=randomized controlled trial; sPGA=static Physician’s Global Assessment; TB=tuberculosis; TEAE=treatment-emergent adverse event; TNF=tumor necrosis factor; UST=ustekinumab.
EU Indications and Important Safety Information for SKYRIZI
INDICATIONS1
Skyrizi (risankizumab) is indicated for the treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic therapy.
Skyrizi, alone or in combination with methotrexate (MTX), is indicated for the treatment of active psoriatic arthritisin adults who have had an inadequate response or who have been intolerant to one or more disease-modifying antirheumaticdrugs (DMARDs).
Skyrizi is indicated for the treatment of adult patients with moderately to severely active Crohn’s disease who have had an inadequate response to, lost response to, or were intolerant to conventional therapy or a biologic therapy.
IMPORTANT SAFETY INFORMATION1
Risankizumab is contraindicated in patients hypersensitive to the active substance or to any of the excipients, and in patients with clinically important active infections (e.g. active tuberculosis). Risankizumab may increase the risk of infection. In patients with a chronic infection, a history of recurrent infection, or known risk factors for infection, risankizumab should be used with caution. Treatment with risankizumab should not be initiated in patients with any clinically important active infection until the infection resolves or is adequately treated.
Patients treated with risankizumab should be instructed to seek medical advice if signs or symptoms of clinically important chronic or acute infection occur. If a patient develops such an infection or is not responding to standard therapy for the infection, the patient should be closely monitored and risankizumab should not be administered until the infection resolves.
Prior to initiating treatment with risankizumab, patients should be evaluated for tuberculosis (TB) infection. Patients receiving risankizumab should be monitored for signs and symptoms of active TB. Anti-TB therapy should be considered prior to initiating risankizumab in patients with a past history of latent or active TB in whom an adequate course of treatment cannot be confirmed.
Prior to initiating therapy with risankizumab, completion of all appropriate immunisations should be considered according to current immunisation guidelines. If a patient has received live vaccination (viral or bacterial), it is recommended to wait at least 4 weeks prior to starting treatment with risankizumab. Patients treated with risankizumab should not receive live vaccines during treatment and for at least 21 weeks after treatment.
If a serious hypersensitivity reaction occurs, administration of risankizumab should be discontinued immediately and appropriate therapy initiated.
The most frequently reported adverse reactions were upper respiratory infections (from 13% in psoriasis to 15.6% in Crohn’s disease). Commonly (≥ 1/100 to < 1/10) reported adverse reactions included tinea infections, headache, pruritus, rash, fatigue, and injection site reactions.
This is not a complete summary of all safety information. Please see the SmPC for complete prescribing information.
Find out more about SKYRIZI
References
- SKYRIZI [Summary of Product Characteristics]. AbbVie Ltd; March 2024.
- Gaffen SL, Jain R, Garg AV, Cua DJ. The IL-23-IL-17 immune axis: from mechanisms to therapeutic testing. Nature Rev Immunol. 2014;14(9):585-600. doi:10.1038/nri3707
- Gooderham MJ, Papp KA, Lynde CW. Shifting the focus – the primary role of IL-23 in psoriasis and other inflammatory disorders. J Eur Acad Derm Venereol. 2018;32(7):1111-1119. doi:10.1111/jdv.14868
- Girolomoni G, Strohal R, Puig L, et al. The role of IL-23 and the IL-23/TH17 immune axis in the pathogenesis and treatment of psoriasis. J Eur Acad Dermatol Venereol. 2017;31(10):1616-1626. doi:10.1111/jdv.14433
- Lynde CW, Poulin Y, Vender R, Bourcier M, Khalil S. Interleukin 17A: toward a new understanding of psoriasis pathogenesis. J Am Acad Dermatol. 2014;71(1):141-150. doi:10.1016/j.jaad.2013.12.036
- Hawkes JE, Chan TC, Krueger JG. Psoriasis pathogenesis and the development of novel targeted immune therapies. J Allergy Clin Immunol. 2017;140(3):645-653. doi: 10.1016/j.jaci.2017.07.004
- Leung S, Liu X, Fang L, Chen X, Guo T, Zhang J. The cytokine milieu in the interplay of pathogenic Th1/Th17 cells and regulatory T cells in autoimmune disease. Cell Mol Immunol. 2010;7(3):182-189. doi:10.1038/cmi.2010.22
- Zúñiga LA, Jain R, Haines C, Cua DJ. Th17 cell development: from the cradle to the grave. Immunol Rev. 2013;252(1):78-88. doi:10.1111/imr.12036
- Lee JS, Tato CM, Joyce-Shaikh B, et al. Interleukin-23-independent IL-17 production regulates intestinal epithelial permeability. Immunity. 2015;43(4):727-738. doi:10.1016/j.immuni.2015.09.003
- Patel DD, Kuchroo VK. Th17 cell pathway in human immunity: lessons from genetics and therapeutic interventions. Immunity. 2015;43(6):1040-1051. doi:10.1016/j.immuni.2015.12.003
- Pastor-Fernández G, Mariblanca IR, Navarro MN. Decoding IL-23 signaling cascade for new therapeutic opportunities. Cells. 2020;9(9):2044. doi:10.3390/cells9092044