
COMBIGAN® offered an effective step-up in patients uncontrolled on β-blockers who required additional IOP-lowering*,1,2
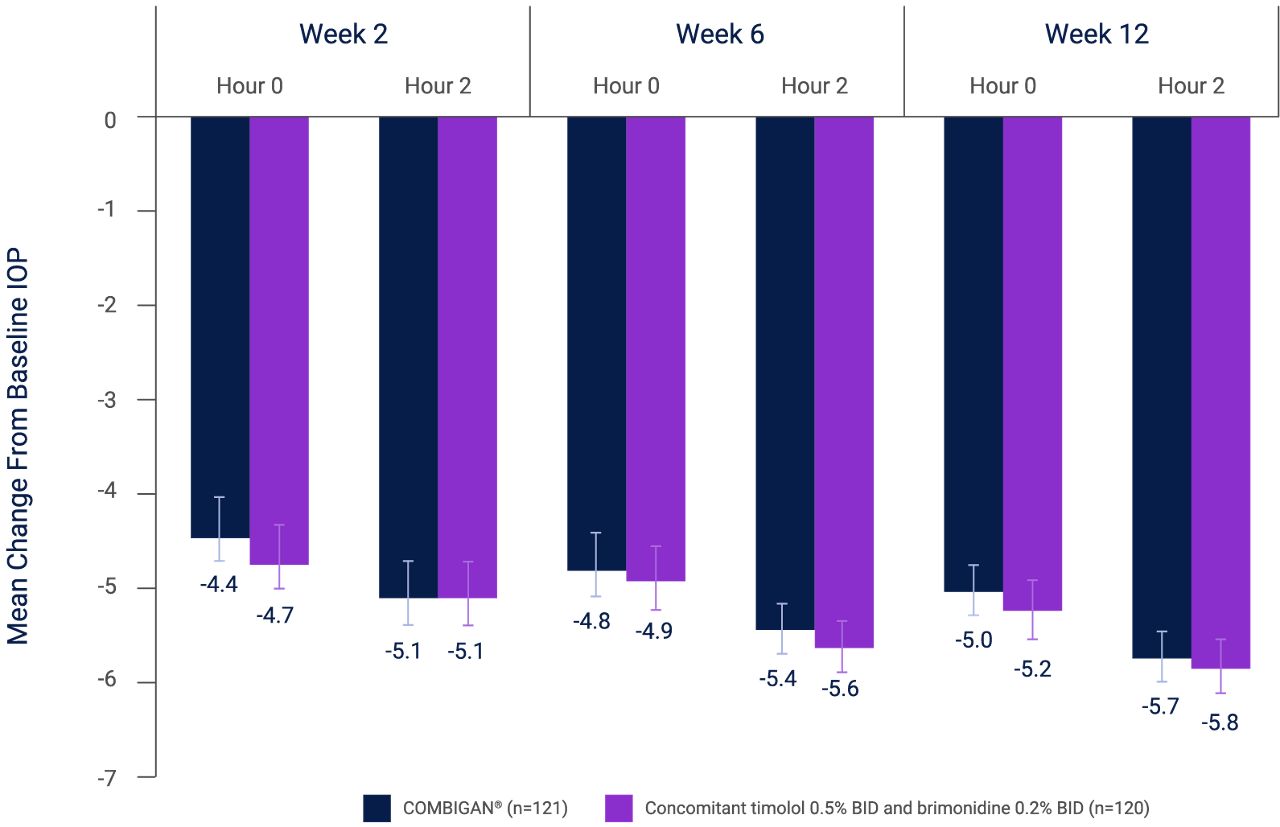
Twice-daily COMBIGAN® achieved a 4.4–5.7 mmHg reduction in IOP at 12 weeks from baseline (25.0 mmHg at hour 0, 22.7 mmHg at hour 2, n=121) in patients previously treated with a β-blocker who required additional IOP reduction vs. a 4.7–5.8 mmHg reduction with concomitant therapy of twice-daily brimonidine 0.2% and twice-daily timolol 0.5% from baseline (24.9 mmHg at hour 0, 22.6 mmHg at hour 2, n=120).*,2 No significant differences in IOP reduction were seen among the treatment groups.2
* Results of a randomized, multicenter, double-masked, parallel-group study involving 355 patients with inadequate IOP control (IOP from 22–34 mmHg) after ≥3 weeks of run-in on any monotherapy. Patients were treated with twice-daily COMBIGAN® or twice-daily concomitant brimonidine 0.2% and timolol 0.5%. Data is specific to patients on prior β-blocker therapy based off a subgroup analysis (n=241).2 The overall incidence of AEs, regardless of causality, was comparable between groups (30.3% in the combination group and 24.6% in the concomitant group). Most AEs were mild or moderate in severity. One or more treatment-related AEs, identified by the investigator as possibly, probably, or definitely related to treatment, were reported for 20.2% of patients in the combination group and 14.2% in the concomitant group (p=0.126). Ocular pain, ocular pruritus, and headache were the most commonly reported treatment-related AEs. There were no significant between-group differences in the incidence of any particular AE. No unexpected or serious treatment-related AEs were reported.2
COMBIGAN® UD (brimonidine 0.2%/timolol 0.5%))
Mean change from baseline IOP on run-in monotherapy for the subgroup of patients whose run-in medication was β-blockersGON[K]
Adapted from Goni et al. 2005.2
BID, twice daily; IOP, intraocular pressure; SEM, standard error of the mean.
Analysis of results for the intent-to treat population is shown. Baseline mean IOP was comparable between treatments (at hour 0: combination, 25.0 mmHg; concomitant, 24.9 mmHg, p=0.985; at hour 2: combination, 22.7 mmHg; concomitant, 22.6 mmHg, p=0.802). Differences between treatments (combination – concomitant) ranged from 0.11–0.33 mmHg; none were statistically significant (p≥0.374). Results were similar for the per-protocol population. Error bars represent SEM.2
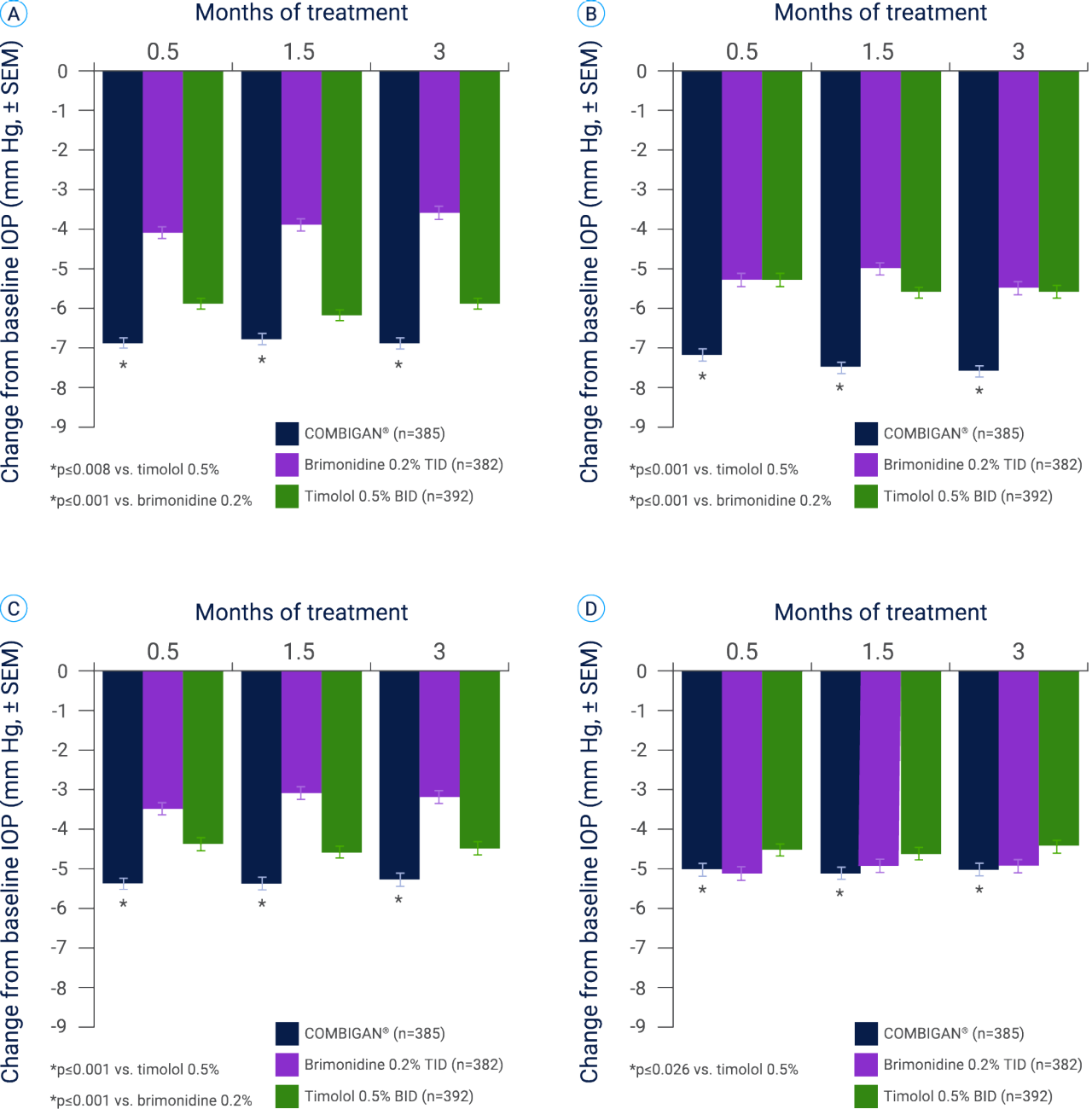
COMBIGAN® could offer greater IOP reduction than its individual components used alone3
Patients receiving COMBIGAN® (n=385) demonstrated significantly better diurnal IOP control vs. brimonidine 0.2% (n=382; p<0.001) or timolol 0.5% (n=392; p≤0.026) monotherapy at all diurnal time points, except 5 pm for brimonidine 0.2%.**,3
** Results of a multicenter, double-masked study comparing the safety and IOP-lowering efficacy of twice-daily COMBIGAN® vs. each drug used as a monotherapy (thrice-daily brimonidine 0.2%, or twice-daily timolol 0.5%) in patients with glaucoma or OHT, (N=1,159).3 The overall incidence of AEs in the COMBIGAN® BID group (n=211/385, 54.8%) was similar to the incidence in the timolol 0.5% BID group (n=205/392, 52.3%; p=0.483) and lower than the incidence in the brimonidine 0.2% TID group (n=245/382, 64.1%; p=0.008). The most common AEs regardless of causality in the COMBIGAN® BID group, brimonidine 0.2% TID group, and timolol 0.5% BID groups, respectively, were ocular burning (n=38/385, 9.9%; n=21/382, 5.5%; n=45/392, 11.5%), ocular stinging (n=21/385, 5.5%; n=7/382, 1.8%; n=26/392, 6.6%), eye pruritus (n=9/385, 2.3%; n=25/382, 6.5%; n=9/392, 2.3%), asthenia (n=9/385, 2.3%; n=13/382, 3.4%; n=2=392, 0.5%), oral dryness (n=7/385, 1.8%; n=36/382, 9.4%; n=2/392, 0.5%), conjunctival folliculitis (n=5/385, 1.3%; n=16/382, 4.2%; n=2/392; 0.5%), and allergic conjunctivitis (n=4/385, 1.0%; n=17/382, 4.5%; n=0/392, 0%).CRA[I] There were fewer serious AEs among patients in the COMBIGAN® group (n=3/385, 0.8%) than in the brimonidine 0.2% group (n=11/382, 2.9%; p=0.030) or the timolol 0.5% group (n=11/392, 2.8%; p=0.034).3
Mean IOP change from baseline at (A) 8 am, (B) 10 am, (C) 3 pm, and (D) 5 pm3
Adapted from Craven et al. 2005.3
IOP, intraocular pressure; SEM, standard error of the mean.
Error bars represent standard error of the mean.
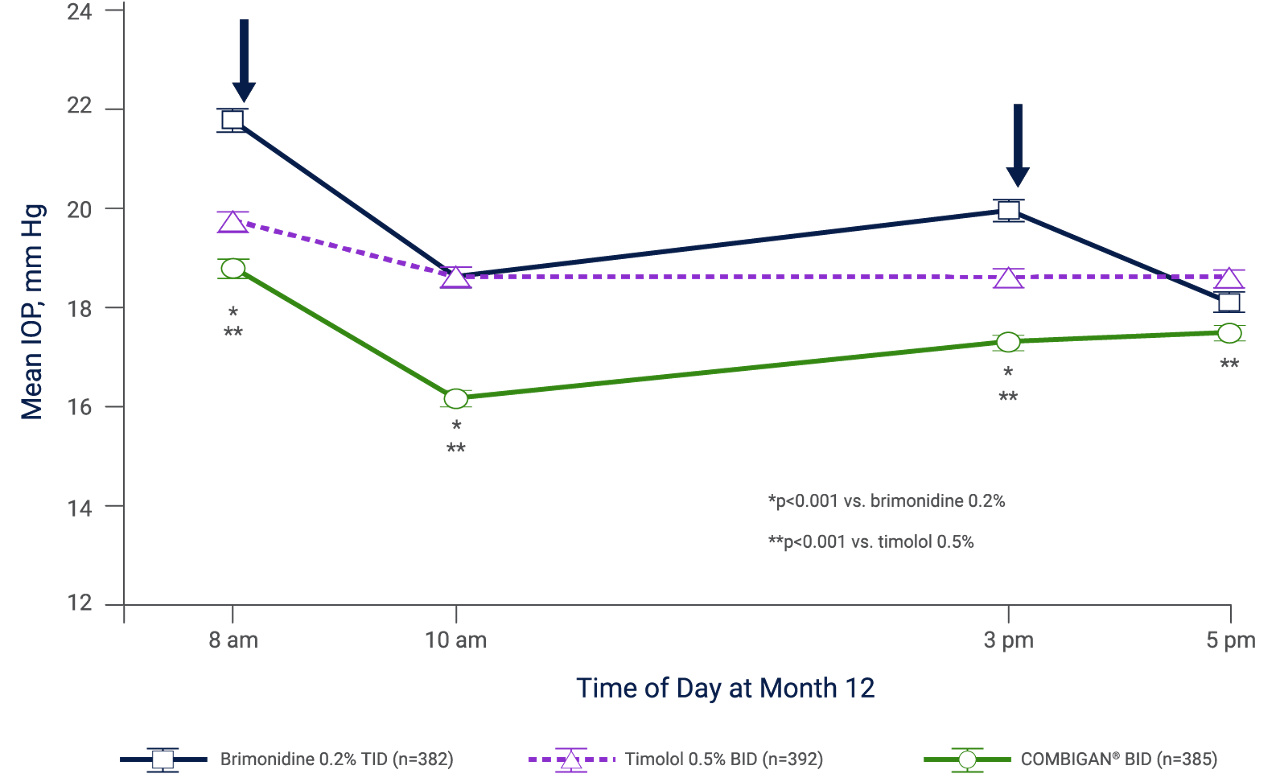
Twice-daily COMBIGAN® (n=385) demonstrated a clinically meaningful improvement in diurnal IOP at all time points (except for 5 pm for brimonidine 0.2%) vs. twice-daily timolol 0.5% (n=392) and thrice-daily brimonidine 0.2% (n=382) when administered as a monotherapy.†,4
† Results of two identical, 12-month, randomized, double-masked multicenter trials evaluating the IOP-lowering efficacy and safety of twice-daily COMBIGAN® (n=385) vs. the component medications, brimonidine 0.2% TID (n=382) and timolol 0.5% BID (n=392).4 Compared with patients receiving brimonidine 0.2% TID monotherapy, patients receiving the fixed combination had a lower incidence of 1 or more treatment-related AEs (53.0% vs. 62.8%; p=0.006), discontinuations due to AEs (14.3% vs. 30.6%; p<0.001), and at least a 1-grade increase in severity in biomicroscopic findings (54.8% vs. 62.8%; p=0.02). The only treatment-related individual AEs that occurred in at least 10% of patients in any group and showed a significant difference among groups were conjunctival hyperemia and eye pruritus.4
Mean IOP at 8 am, 10 am, 3 pm, and 5 pm at Month 12†,4
Adapted from Sherwood et al. 2006.4
BID, twice daily; IOP, intraocular pressure; SEM, standard error of the mean; TID, three times daily.
Analysis is based on the intention-to-treat patient population with last observation carried forward for missing values. Similar results were obtained using observed values in the per-protocol patient population. Arrows indicate administration of the morning and afternoon doses. Brimonidine was given as 0.2% brimonidine tartrate; timolol as 0.5% timolol maleate. Error bars represent SEM.4
COMBIGAN® is indicated for the reduction of intraocular pressure (IOP) in patients with chronic open-angle glaucoma or ocular hypertension who are insufficiently responsive to topical beta-blockers.1
Based on 12-month clinical data, the most commonly reported ADRs were conjunctival hyperemia (approximately 15% of patients) and burning sensation in the eye (approximately 11% of patients). The majority of these cases was mild and led to discontinuation rates of only 3.4% and 0.5% respectively.1
Please refer to COMBIGAN® Summary of Product Characteristics for further information on adverse events.
ADR, adverse drug reaction; AE, adverse event; BID, twice-daily; IOP, intraocular pressure; OAG, open-angle glaucoma; OHT, ocular hypertension; TID, thrice-daily.
COMBIGAN® (brimonidine 0.2%/timolol 0.5%). Summary of Product Characteristics. 2022.
Goni F et al. European Journal of Ophthalmology 2005; 15(5): 581–590.
Craven E et al. J Ocul Pharmacol Ther 2005; 21: 337–348.
Sherwood MB, et al. Arch Ophthalmol. 2006; 124(6): 1230–1238.

