
ALPHAGAN® 0.2%® (brimonidine tartrate ophthalmic solution)
ALPHAGAN® 0.2% is indicated for the reduction of elevated intraocular pressure (IOP) in patients with open angle glaucoma or ocular hypertension:ALP[G]
− as monotherapy in patients in whom topical beta-blocker therapy is contraindicated
− as adjunctive therapy to other intraocular pressure lowering medications when the target IOP is not achieved with a single agent
ALPHAGAN® 0.2% (brimonidine ophthalmic solution)
ALPHAGAN® 0.2% demonstrated a rapid onset of action in its two
1-year studiesALP[B]
ALPHAGAN® 0.2% has a rapid onset of action, with peak ocular hypotensive effect seen at two hours post-dosing. In two 1-year studies, twice-daily ALPHAGAN® 0.2%, with 12-hours between doses, lowered IOP by mean values of approximately 4–6 mmHg.ALP[B],KAT[A,M]
Effect of ALPHAGAN® 0.2% and timolol 0.5% at peak (2 hours after morning instillation of drugs) in two long-term clinical studies, with combined data from both studies*,KAT[L]
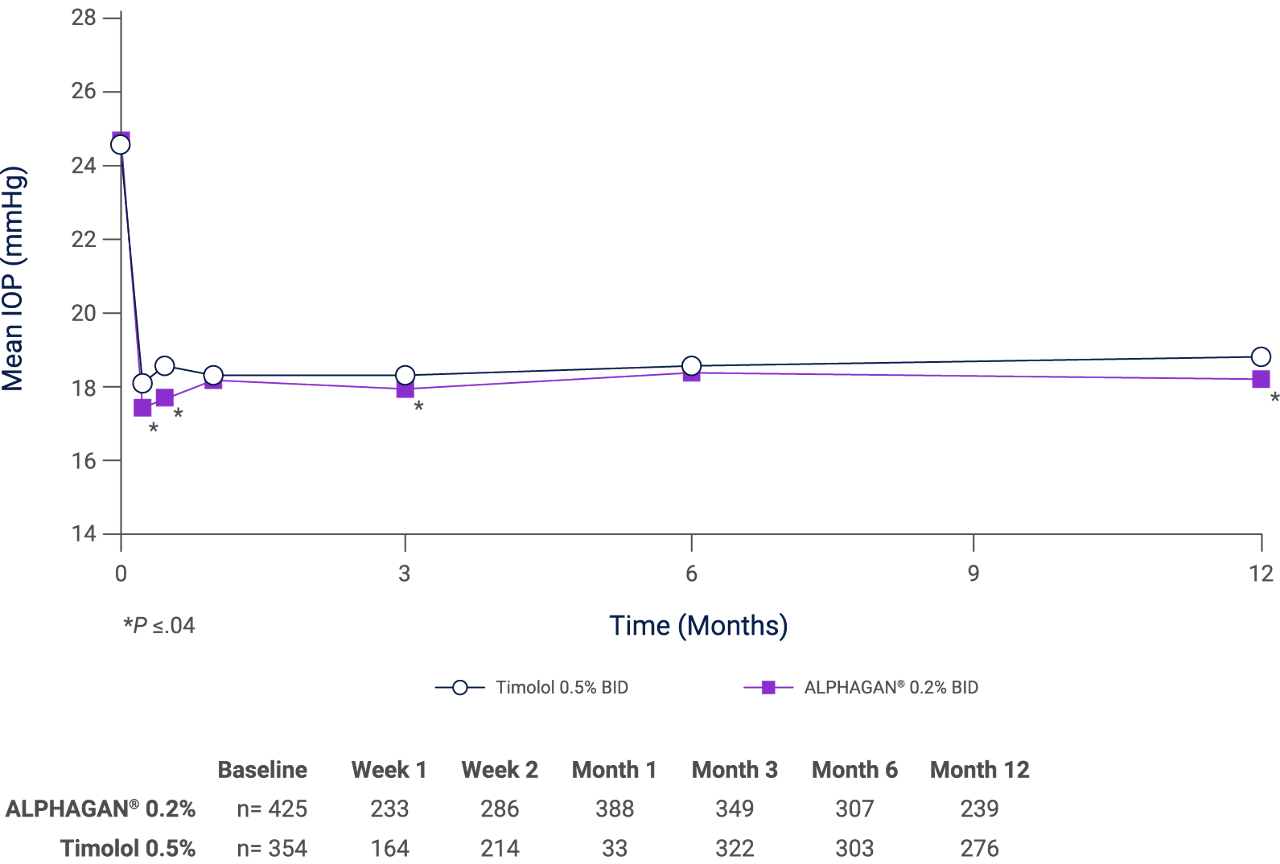
Adapted from Katz L et al. 1999.KAT[L]
* Statistically significantly lower intraocular pressure with ALPHAGAN® 0.2% at week 1 (p=0.011), week 2 (p=0.001), month 3 (p=0.043), and month 12 (p=0.031) N numbers used for statistical analysis at each time point are presented in tabular format.KAT[L]
BID, twice daily; IOP, intraocular pressure.
Results from two multicentre, randomized, double-masked, parallel-group, active-controlled comparisons conducted in the United States (40 sites), Canada (7 sites), Australia (2 sites) and Israel (2 sites). Patients with POAG or OHT (N=837) were randomized to receive 1 drop of either brimonidine tartrate 0.2% (n=466) or timolol maleate 0.5% (n=371) ophthalmic solutions instilled in each eye twice daily at 12-hour intervals (morning and evening) for 12 months.KAT[C,D,M] Please refer to the ‘What is the safety profile of ALPHAGAN® 0.2%?’ section from the navigation bar for a safety summary from this pivotal trial. ALPHAGAN® 0.02% is licensed for a reduction of elevated IOP in patients with OAG or OHT as monotherapy in patients in whom topical beta-blocker therapy is contraindicated.ALP[F]
If monotherapy is well tolerated and effective, but has not lowered IOP to the target pressure, the EGS recommend adding another drug of a different class as adjunctive therapyEGS[A]
When used adjunctively to latanoprost 0.005%, ALPHAGAN® 0.2% (n=22) provided greater mean IOP reduction vs. dorzolamide 2%/timolol 0.5% fixed-combination (n=18) at 6 weeks (34.7% [9.2±3.6 mmHg] vs. 26.1% [6.7±3.5 mmHg], p=0.024) and 12 weeks (33.9% [9.0±4.5 mmHg] vs. 25.3% [6.5±3.6 mmHg], p=0.044) from baseline (26.4±5.0 mmHg in ALPHAGAN®/latanoprost group and 25.6±2.9 mmHg in the dorzolamide/timolol group).*,ZAB[D,G]
*Results of a double-masked, randomized, multicenter trial comparing the IOP-lowering efficacy of dual therapy with twice-daily ALPHAGAN® 0.2% and once-daily latanoprost 0.005% with vehicle solution (n=22) with twice-daily fixed-combination of dorzolamide 2%/timolol 0.5% (n=18) in patients with OAG, OHT, pigmentary glaucoma or pseudoexfoliative glaucoma (N=40).ZAB[B,G,I] The regimen was safe and well tolerated, and few patients discontinued treatment because of adverse events. One patient in the ALPHAGAN® 0.2%/latanoprost group withdrew because of dry eyes and another because of chest pain unrelated to the study medications. Three discontinuations in the timolol/dorzolamide group were due to dry mouth, allergy, and nausea with lack of appetite, all considered treatment related.ZAB[K]
Mean IOP reduction (peak drug effect) at each visit in study 1ZAB[H]

Adapted from Zabriskie N and Netland P 2003.ZAB[H]
In a second study of similar design, the mean peak IOP reduction at month 1 was 10.6 mmHg (39.0%) with ALPHAGAN® (n=12) and latanoprost 0.005% and 6.3 mmHg (25.1%) with timolol 0.5%/dorzolamide 2% (n=11; p=0.001). After 3 months, reductions were 9.1 mmHg (33.4%) and 6.6 mmHg (26.3%) (p=0.047), further demonstrating a greater mean IOP reduction when ALPHAGAN® 0.2% was used adjunctively to latanoprost 0.005% than dorzolamide 2%/timolol 0.5% fixed combination.*,ZAB[A]
*Results of a double-masked, randomized, multicenter trial comparing the IOP-lowering efficacy of dual therapy with twice-daily ALPHAGAN® 0.2% and once-daily latanoprost 0.005% in the evening (n=12) with twice-daily fixed-combination of dorzolamide 2%/timolol 0.5% and vehicle once daily in the evening (n=11) in patients with OAG or OHT (N=23).ZAB[C,F,I] The regimen was safe and well tolerated - one patient instilling timolol/dorzolamide left prematurely because of palpitations unrelated to treatment.ZAB[K]
Mean IOP reduction (peak drug effect) at each visit in study 2ZAB[J]
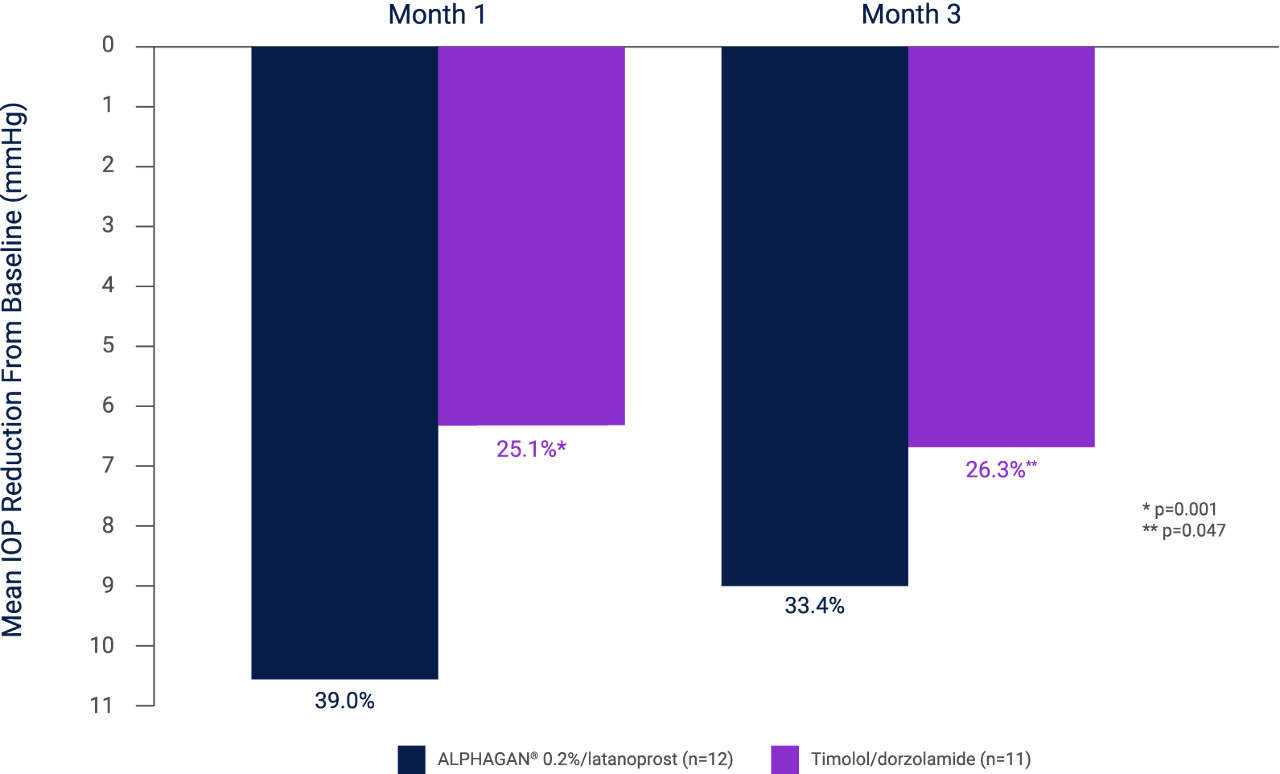
Adapted from Zabriskie N and Netland P 2003.4
ALPHAGAN® 0.2% has demonstrated IOP-lowering efficacy in short and long-term clinical trials2,5–8
ALPHAGAN® 0.2% (n=1,281) demonstrated similar IOP-lowering efficacy to timolol 0.5% (n=1,174) in a meta-analysis of 8 trials, ranging from 1–36 months follow up.5
Please refer to ALPHAGAN® 0.2% Summary of Product Characteristics for further adverse events and safety information.
ALPHAGAN® 0.2% is indicated for the reduction of elevated intraocular pressure (IOP) in patients with open angle glaucoma or
ocular hypertension.1
- as monotherapy in patients in whom topical beta-blocker therapy is contraindicated
- as adjunctive therapy to other intraocular pressure lowering medications when the target IOP is not achieved with a single agent
The most commonly reported ADRs are oral dryness, ocular hyperemia and burning/stinging, all occurring in 22 to 25% of patients. They are usually transient and not commonly of a severity requiring discontinuation of treatment. Symptoms of ocular allergic reactions occurred in 12.7% of subjects (causing withdrawal in 11.5% of subjects) in clinical trials with the onset between 3 and 9 months in the majority of patients.1
ADR, adverse drug reaction; EGS, European glaucoma society; IOP, intraocular pressure.
1. ALPHAGAN® (brimonidine tartrate ophthalmic solution) 0.2%. Summary of Product Characteristics. 2020.
2. Katz L. Am J Ophthalmol 1999; 127: 20–26.
3. European Glaucoma Society. Terminology and Guidelines for Glaucoma. 5th edition. EGS. 2020.
4. Zabriskie N & Netland P. Adv Ther 2003; 20: 92–100.
5. Loon S et al. Clin. Experiment Ophthalmol 2008; 36: 281–289.
6. Schuman J et al. Arch Ophthalmol 1997; 115: 847–852.
7. Le Blanc R. Ophthalmology 1998; 105: 1960–1967.
8. Melamed S & David R. Clin Ther 2000; 22: 103–111.

