
ALPHAGAN® P 0.1% or 0.15% (brimonidine ophthalmic solution)
ALPHAGAN® P 0.1% or 0.15% is indicated for the reduction of elevated intraocular pressure (IOP) in patients with open angle glaucoma or ocular hypertension1
ALPHAGAN® 0.2% (brimonidine ophthalmic solution)
ALPHAGAN® P 0.1% or 0.15% has demonstrated IOP-lowering efficacy equivalent to ALPHAGAN® 0.2%.1
A clinical study evaluating the safety, efficacy, and acceptability of thrice-daily ALPHAGAN® P 0.1% or 0.15% compared with ALPHAGAN® 0.2% indicated a comparable IOP-lowering effect, lowering IOP in patients with OAG or OHT by approximately 2–6 mmHg.1
As monotherapy, ALPHAGAN® P 0.1% demonstrated IOP-lowering efficacy during the diurnal/wake period
over 4 weeks2
Over 4 weeks, ALPHAGAN® P 0.1% reduced diurnal IOP by 2.4±1.4 mmHg while sitting, and 1.9±1.3 mmHg while supine from baseline (19.2±2.7 mmHg and 23.1±2.1 mmHg, respectively; N=15), p<0.01.*,2
*Results of a 4-week prospective open-label experimental study investigating the effect of thrice-daily ALPHAGAN® P 0.1% on IOP during the diurnal and nocturnal/sleep period in patients with newly diagnosed open-angle glaucoma or OHT. Thrice-daily ALPHAGAN® P 0.1% did not significantly reduce IOP during the nocturnal period, (N=15).2 Safety findings were not reported.
If monotherapy is well tolerated and effective, but has not lowered IOP to the target pressure, the EGS recommend adding another drug of a different class as adjunctive therapy3
As adjunctive therapy to a PGA, ALPHAGAN® P 0.1% could provide equivalent or greater IOP reduction vs. brinzolamide 1%4
Over A combination of ALPHAGAN® P 0.1% and latanoprost 0.005% (n=20) provided greater IOP lowering than brinzolamide 1%/latanoprost 0.005% (n=20) from baseline (19.4 mmHg and 18.9 mmHg, respectively) at 10 am with a reduction of 4.8 mmHg vs. 16 mmHg; p<0.001.*,4
A combination of ALPHAGAN® P 0.1% and latanoprost 0.005% (n=20) provided greater IOP lowering than brinzolamide 1%/latanoprost 0.005% (n=20) from baseline (19.1 mmHg and 19.6 mmHg, respectively) at 4 pm with a reduction of 2.8 mmHg vs. 1.8 mmHg; p=0.05.*,4
A combination of ALPHAGAN® P 0.1% and latanoprost 0.005% (n=20) provided an equivalent IOP-lowering effect brinzolamide 1%/latanoprost 0.005% (n=20) from baseline (20.4 mmHg and 21.0 mmHg) at 8 am with a reduction of 2.2 mmHg vs. 2.8 mmHg; p=0.716.*,4
Additional studies are needed to further evaluate these drugs as adjunctive therapy to PGAs as this study was limited by its small sample size and single site location.4
*Results of a 3-month randomized, single-center, investigator-masked, parallel- group clinical study evaluating the efficacy and tolerability of thrice-daily ALPHAGAN® P 0.1% (n=20) in comparison to thrice-daily brinzolamide 0.1% (n=20) when used as adjunctive therapy to once-daily latanoprost 0.005% in patients with glaucoma or OHT. Additional studies are needed as this study was limited by its small sample size and single site location.4 There was no significant difference between groups in the overall incidence of treatment-related AEs or in the incidence of any individual treatment-related AE (brimonidine, n=4/20; brinzolamide, n=3/20). The most common treatment-related AEs were ocular hyperemia and eye pain.4
As adjunctive therapy to a PGA, ALPHAGAN® P 0.15% could also provide greater IOP reduction vs. dorzolamide 2% or brinzolamide 1%5
The mean IOP and mean change from baseline IOP was significantly greater in patients treated with adjunctive ALPHAGAN® P 0.15% (n=41) than in either the dorzolamide 2% group (n=40) or the brinzolamide 1% (n=39) group at 10 am and 4 pm at months 1 and 4 (p<0.001).†,5
After 4 months of adjunctive therapy, the mean IOP reduction from baseline at 10 am and 4 pm was 4.8 mmHg (21%) and 3.8 mmHg (19%) with ALPHAGAN® P 0.15% (n=41), 3.4 mmHg (16%) and 2.8 mmHg (14%) with dorzolamide 2% (n=40), and 3.4 mmHg (16%) and 2.6 mmHg (13%) with brinzolamide 1% (n=39); (p<0.001 for ALPHAGAN® P 0.15% vs. dorzolamide 2% and brinzolamide 1% at each time point).†,5
Additional studies are required to determine whether greater IOP reduction is achieved with ALPHAGAN® P 0.15% when added to a PGA.5
All drugs were dosed thrice daily – markets must check with their local labels whether this falls within license in their markets.
†Results of a randomized, controlled, investigator-masked, single-site, parallel-group clinical trial comparing the IOP-lowering efficacy of adjunctive thrice-daily ALPHAGAN® P 0.15%, dorzolamide 2%, and brinzolamide 1% in 120 eyes of 120 patients with OAG or OHT who had inadequate IOP control after at least 6 weeks of monotherapy with a once-daily PGA (bimatoprost, latanoprost, or travoprost). Further studies are needed to evaluate the relative long-term efficacy and tolerability of these medications as adjunctive therapy to a PGA.5 All of the study drugs were well tolerated. None of the 120 enrolled patients discontinued from the study for safety or tolerability reasons. At the end of the study, however, 1 patient (2.4%) in the brimonidine group, 2 patients (5.0%) in the dorzolamide group, and 2 patients (5.1%) in the brinzolamide group requested different therapy because of ocular discomfort.5
Mean change from baseline IOP at 10 am and 4 pm after 1 and 3 months of treatment5
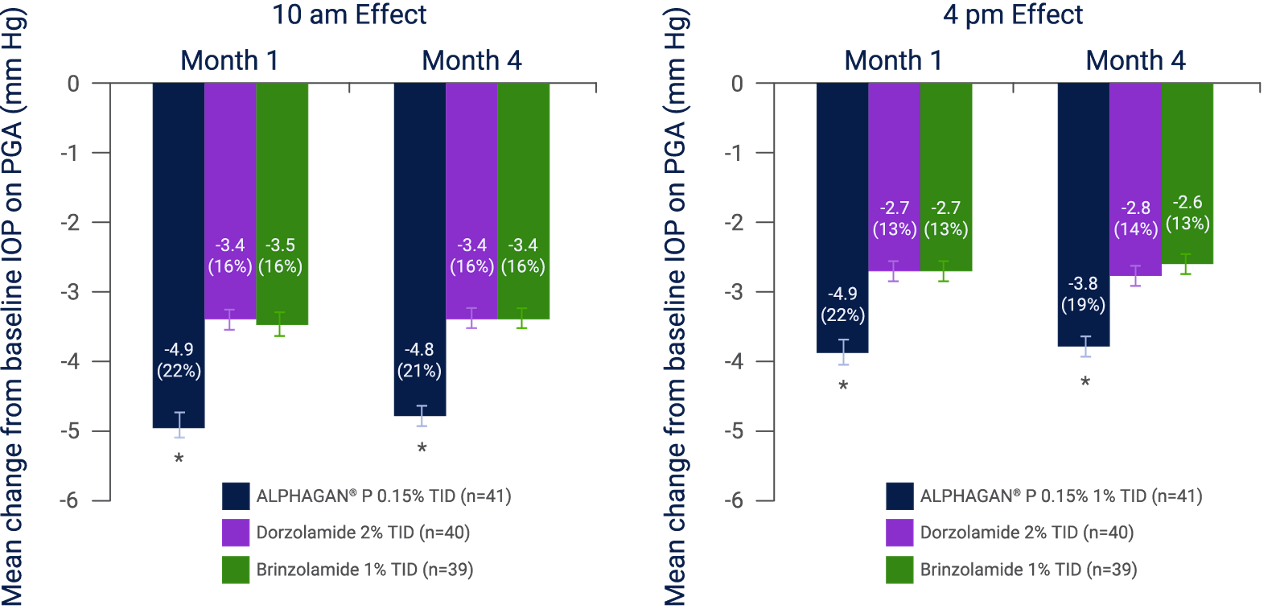
Adapted from Bournia T and Lai J. 2009.5
Error bars represent SEM.
IOP, intraocular pressure; PGA, prostaglandin analog; SEM, standard error of the mean.
*p<0.001 vs. dorzolamide and brinzolamide.
Please refer to ALPHAGAN® P 0.1% or 0.15% Summary of Product Characteristics for further adverse event and safety information.
ALPHAGAN® P 0.1% or 0.15% is indicated for the reduction of elevated intraocular pressure (IOP) in patients with open angle glaucoma or ocular hypertension.1
Most common adverse reactions occurring in approximately 5% to 20% of patients receiving brimonidine ophthalmic solution (0.1%–0.2%) included allergic conjunctivitis, burning sensation, conjunctival folliculosis, conjunctival hyperemia, eye pruritus, hypertension, ocular allergic reaction, oral dryness, and visual disturbance.1
ADR, adverse drug reaction; EGS, European glaucoma society; IOP, intraocular pressure; PGA, prostaglandin analog; OAG, open-angle glaucoma; OHT, ocular hypertension..
1. ALPHAGAN® P (brimonidine tartrate ophthalmic solution) 0.1% or 0.15%. Prescribing Information. 2013.
2. Liu J et al. Ophthalmology 2010; 117(11): 2075–2079.
3. European Glaucoma Society. Terminology and Guidelines for Glaucoma. 5th edition. EGS. 2020.
4. Day D and Hollander D. Curr Med Res Opin 2008; 24(5): 1435–1442.
5. Bournias T and Lai J. Ophthalmology 2009; 116(9): 1719–1724.
[Countries to include local adverse event reporting information and link to the prescribing information]

