GANFORT® (bimatoprost/timolol ophthalmic solution)
GANFORT® UD is indicated for the reduction of intraocular pressure in adult patients with open-angle glaucoma or ocular hypertension who are insufficiently responsive to topical beta-blockers or prostaglandin analogs.1
GANFORT® UD (bimatoprost/timolol ophthalmic solution 0.03/0.5%)
GANFORT® UD offers an established tolerability profile in a preservative-free formulation1,2
The majority of adverse reactions reported in clinical studies using GANFORT® UD were ocular, mild in severity, and none were serious.1 They were limited to those earlier reported for either GANFORT® MD or the single active substances bimatoprost and timolol.1
The most commonly reported adverse reaction in clinical studies of both GANFORT® and GANFORT® UD administered once daily was conjunctival hyperemia (mostly trace to mild and thought to be of a non-inflammatory nature. This occurred in 26% of MD patients and 21% of UD patients, and led to discontinuation in 1.5% of MD patients and 1.4% of UD patients.1,3
Please refer to GANFORT® UD Summary of Product Characteristics for further safety and adverse event information.
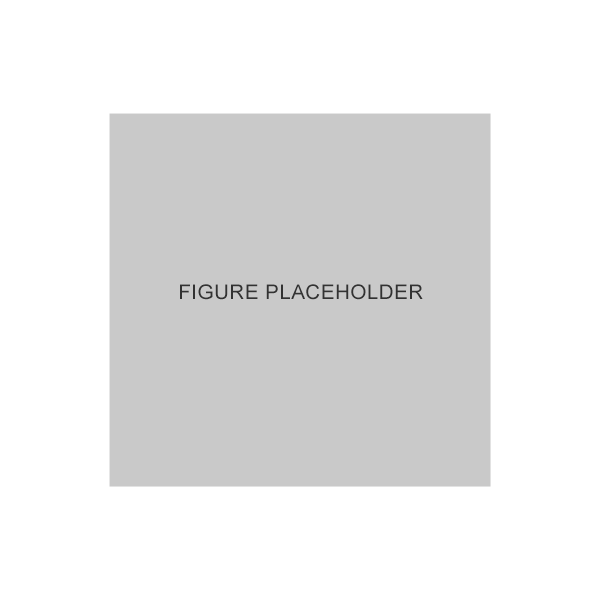
Very common and common adverse events associated with GANFORT® UD1
| System Organ class | Frequency | Adverse reaction |
|---|---|---|
| Nervous system disorders | Common
| Headache |
| Very common | Conjunctival hyperemia | |
| Eye disorders | ||
| Common | Punctuate keratitis, conjunctival irritation, eye pruritus, foreign body sensation, dry eye, erythema of the eyelid, eye pain, photophobia, eye discharge, eyelid pruritus, eyelid edema, eye irritation, lacrimation increased, growth of eyelashes | |
| Skin and subcutaneous tissue disorders | Common | Skin hyperpigmentation (periocular) |
Adapted from GANFORT® UD Summary of Product Characteristics. 2022.1
RCT, randomized controlled trial; UD, unit dose
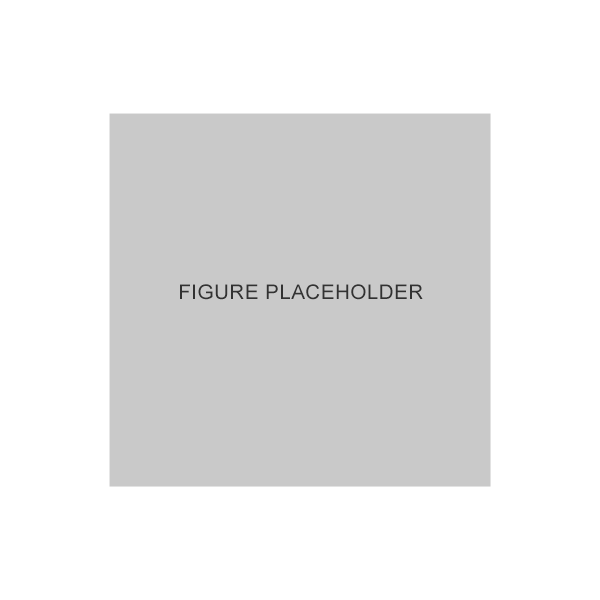
GANFORT® UD could have additional tolerability benefits compared to BAK-preserved treatments*,4
Mean meibomian acinar density was significantly higher in patients treated with GANFORT UD vs. patients treated with preserved PGA FC therapy4
Adapted from Agnifili L et al. 2018.4
*GANFORT® UD vs. PGA FCs, p<0.05.4
Results of a cross-sectional study in which 60 white patients were treated with prostaglandin/timolol fixed combinations, 15 with latanoprost+timolol unfixed combination, and 15 controls were enrolled. Patients underwent the Ocular Surface Disease Index questionnaire, tear film breakup time, corneal staining, Schirmer test I, and IVCM of meibomian glands and goblet cells.4
FC, fixed combination; IVCM, in vivo confocal microscopy; PGA, prostaglandin analog; UD, unit dose.
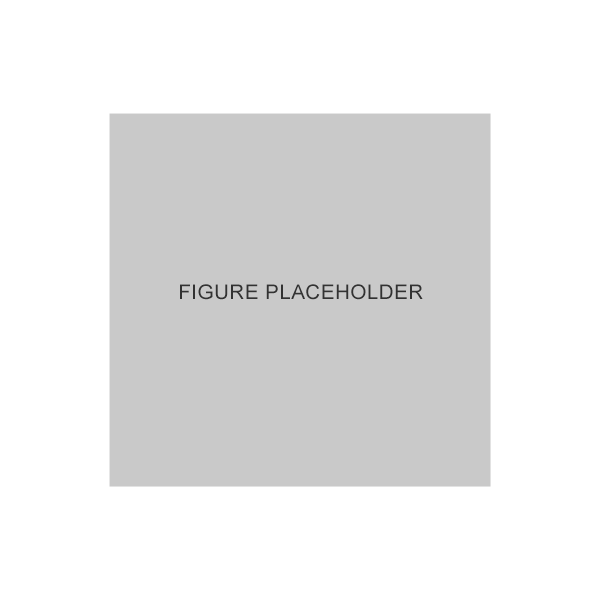
Conjunctival goblet cell density was significantly higher in patients treated with GANFORT UD vs. patients treated with preserved PGA FC therapy4
Adapted from Agnifili L et al. 2018.4
*GANFORT® UD vs. PGA FCs, p<0.05.4
Results of a cross-sectional study in which 60 white patients were treated with prostaglandin/timolol fixed combinations, 15 with latanoprost+timolol unfixed combination, and 15 controls were enrolled. Patients underwent the Ocular Surface Disease Index questionnaire, tear film breakup time, corneal staining, Schirmer test I, and IVCM of meibomian glands and goblet cells.4
FC, fixed combination; IVCM, in vivo confocal microscopy; PGA, prostaglandin analog; UD, unit dose.
Preservative-free GANFORT® UD resulted in fewer changes to the meibomian gland and conjunctival goblet cells than BAK-preserved treatments.*,**,4
*Based on meibomian acinar and conjunctival goblet cell density.4
**These findings should be carefully considered given the role of these structures in the induction of glaucoma-related ocular surface disease.4
Reporting of suspected adverse reactions
GANFORT® single-dose is indicated for the reduction of intraocular pressure (IOP) in patients with open-angle glaucoma or ocular hypertension who are insufficiently responsive to topical beta-blockers or prostaglandin analogs.1
The majority of adverse reactions reported with GANFORT single-dose were ocular, mild in severity and none were serious. Based on a 12-week study of GANFORT single-dose administered once daily, the most commonly reported adverse reaction with GANFORT single-dose was conjunctival hyperaemia (mostly trace to mild and thought to be of a non-inflammatory nature) in approximately 21% of patients and led to discontinuation in 1.4% of patients.1
Please refer to GANFORT® UD Summary of Product Characteristics for further information on adverse events.
BAK, benzalkonium chloride; IOP, intraocular pressure; MD, multi dose; OAG, open-angle glaucoma; OHT, ocular hypertension; UD, unit dose.
EGS – European Glaucoma Society (EGS). Terminology and Guidelines for Glaucoma. Fifth edition. EGS. 2020.
GAN1 - GANFORT® UD (bimatoprost/timolol ophthalmic solution 0.03%/0.5%). Summary of Product Characteristics. 2022.
AGN – Agnifili L et al. J Glaucoma 2018; 27(4): 364–370.