GANFORT® (bimatoprost/timolol ophthalmic solution)
GANFORT® is indicated for the reduction of intraocular pressure in adult patients with open-angle glaucoma or ocular hypertension who are insufficiently responsive to topical beta-blockers or prostaglandin analogs.1
GANFORT® UD (bimatoprost/timolol ophthalmic solution 0.03/0.5%)
GANFORT® offers you and your patients the reassurance of an established tolerability profile1
The adverse reactions reported in clinical studies using GANFORT® were limited to those earlier reported for either of the single active substances bimatoprost and timolol. No new adverse reactions specific for GANFORT® have been observed in clinical studies.1
The majority of adverse reactions reported in clinical studies using GANFORT® were ocular, mild in severity and none were serious. Based on 12-month clinical data, the most commonly reported adverse reaction was conjunctival hyperemia (mostly trace to mild and thought to be of a non-inflammatory nature) in approximately 26% of patients and led to discontinuation in 1.5% of patients.1
Please refer to GANFORT® Summary of Product Characteristics for further information on adverse events.
Very common and common adverse events associated with GANFORT®1
| System Organ class | Frequency | Adverse reaction |
|---|---|---|
| Nervous system disorders | Common
| Headache |
| Very common | Conjunctival hyperemia | |
| Eye disorders | ||
| Common | Punctuate keratitis, corneal erosion, burning sensation, conjunctival irritation, eye pruritus, stinging sensation in the eye, foreign body sensation, dry eye, erythema of eyelid, eye pain, photophobia, eye discharge, visual disturbance, eyelid pruritus, visual acuity worsened, blepharitis, eyelid edema, eye irritation, lacrimation increased, growth of eyelashes | |
| Respiratory, thoracic and mediastinal disorders | Common | Rhinitis |
| Skin and subcutaneous tissue disorders | Common | Blepharal pigmentation, hirsutism, skin hyperpigmentation (periocular) |
Adapted from GANFORT® Summary of Product Characteristics. 2022.1
Please refer to the Summary of Product Characteristics for full details of adverse events associated with GANFORT®.
The tolerability of GANFORT® was highly rated in a multicenter study of 356 patients with OAG or OHT*,**,2
A post hoc subgroup analysis of patients receiving the most common study treatment, GANFORT®, was conducted.2 The subgroup analyses included physician (n=274) and patient (n=267) perceptions of the tolerability of GANFORT®.2
* Defined as a tolerability rating of either good or very good.
** Results of a prospective, non-interventional, multicenter study in Turkey of the IOP-lowering effects of prostaglandin analog/prostamide-containing therapies in previously treated patients with open-angle glaucoma or ocular hypertension, N=358.2 Secondary measures included physician and patient assessment of study treatment tolerability at visit 3.2
IOP, intraocular pressure; OAG, open-angle glaucoma; OHT, ocular hypertension.
GANFORT® demonstrated improved patient centered outcomes vs. previous monotherapy3
GANFORT® may offer improved patient centered outcomes compared to previous monotherapy.3
More than 80% of patients agreed or very much agreed that, in comparison to their previous monotherapy, GANFORT® was associated with improvements in:*,3
• Tolerability and comfort
• Ease of administration
*Results of an observational, multicenter study conducted in China in adults with glaucoma treated with GANFORT® for 1–3 months, N=500. All patients answered a questionnaire concerning their demographic characteristics, history of glaucoma and topical glaucoma treatment, and use of GANFORT®. The primary endpoint was patient satisfaction with GANFORT® assessed on a 10-point scale (1=very dissatisfied, 10=very satisfied).3
Mean satisfaction after switching to GANFORT®3
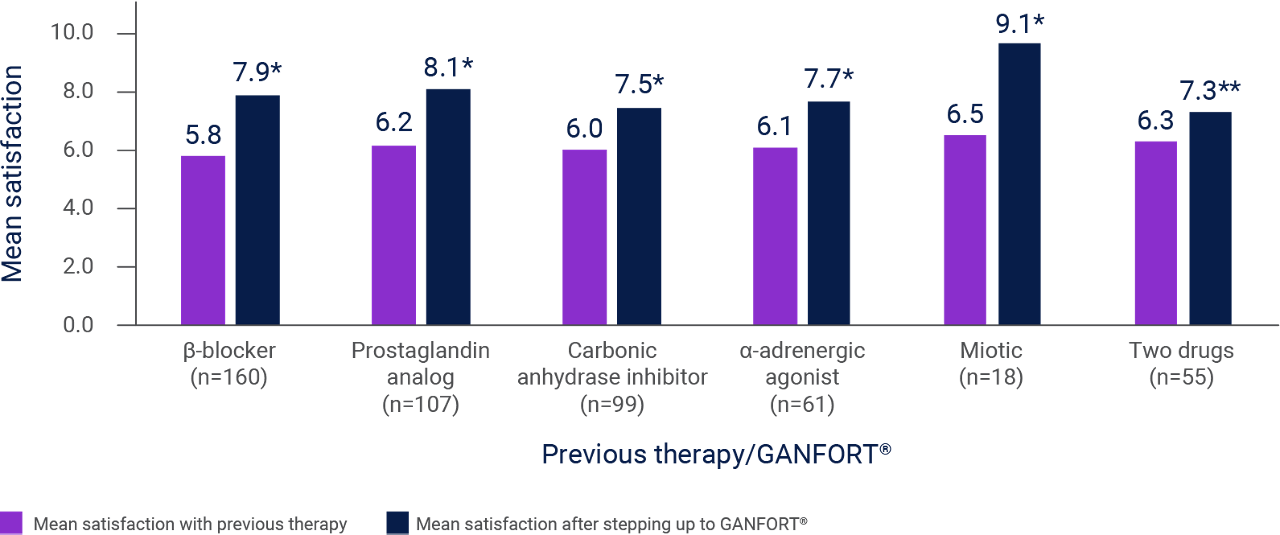
Adapted from Sun X et al. 2017.3
* p<0.0001 vs. previous therapy.
** p=0.0051.
Results of an observational, multicenter study conducted in China in adults with glaucoma treated with GANFORT® for 1–3 months, N=500. All patients answered a questionnaire concerning their demographic characteristics, history of glaucoma and topical glaucoma treatment, and use of GANFORT®. The primary endpoint was patient satisfaction with GANFORT® assessed on a 10-point scale (1=very dissatisfied, 10=very satisfied).3 Patient-reported satisfaction after switching to GANFORT® vs. previous therapy was measured. Any adult outpatient diagnosed with glaucoma who had been using GANFORT® for 1–3 months was eligible. An upper limit of 3 months’ use was chosen to avoid bias, because researchers wanted to compare patients’ satisfaction with GANFORT® vs. their previous treatment, and patients may better remember their satisfaction with a previous regimen when it was used within the past 3 months.3
Reporting of suspected adverse reactions
GANFORT® is indicated for the reduction of intraocular pressure (IOP) in patients with open-angle glaucoma or ocular hypertension who are insufficiently responsive to topical beta-blockers or prostaglandin analogs.1
The majority of adverse reactions reported in clinical studies using GANFORT® were ocular, mild in severity and none were serious. Based on 12-month clinical data, the most commonly reported adverse reaction was conjunctival hyperaemia (mostly trace to mild and thought to be of a non-inflammatory nature) in approximately 26% of patients and led to discontinuation in 1.5% of patients.1
Please refer to GANFORT® Summary of Product Characteristics for further information on adverse events.
IOP, intraocular pressure; OAG, open-angle glaucoma; OHT, ocular hypertension.
1. GANFORT® (bimatoprost/timolol ophthalmic solution 0.03%/0.5%). Summary of Product Characteristics. 2022.
2. Tamcelik N et al. Clin Ophthalmol 2017; 11: 723–731.
3. Sun X et al. Patient Prefer Adherence 2017; 11: 845–852.
05/2024 | ALL-LUM-240002


