GANFORT® (bimatoprost/timolol ophthalmic solution)
GANFORT® UD is indicated for the reduction of intraocular pressure in adult patients with open-angle glaucoma or ocular hypertension who are insufficiently responsive to topical beta-blockers or prostaglandin analogs.1
GANFORT® UD (bimatoprost/timolol ophthalmic solution 0.03/0.5%)
GANFORT® UD offered comparable efficacy to GANFORT® in a multicenter, randomized, parallel-group, 12-week study of patients with OAG or OHT (N=561)2
Mean (±SD) worse eye IOP at each time point through 12 weeks of study in the per-protocol population2

Adapted from Goldberg I et al. 2014.2
IOP, intraocular pressure; OAG, open-angle glaucoma; OHT, ocular hypertension; SD, standard deviation; UD, unit dose.
Results of a multicenter, randomized parallel-group, 12-week study of patients with OAG or OHT randomized to either GANFORT® (n=283) or GANFORT® UD (n=278) conducted at 55 sites in nine countries (Australia, Czech Republic, Germany, Hungary, Israel, Russia, Spain, UK, and the US).2 Both treatments were administered once daily in the morning.2
Treating with GANFORT® UD helped many appropriate patients reach their target IOP3
Switching to GANFORT® UD was associated with significant reductions from baseline over 12 weeks (p<0.0001 vs. baseline, n=1,120).**,3
** Results of a prospective, observational open-label study which patients who switched to GANFORT® UD due to insufficient IOP control on prior therapies or for other reasons as well as treatment-naïve patients. IOP was measured at baseline and at ~12 weeks. Previous therapies reported in ≥5% of patients (per-protocol population) included latanoprost (n=206), PF latanoprost (n=71), bimatoprost (n=135), PF tafluprost (n=133), travoprost (n=123), timolol (n=245), brinzolamide (n=136), travoprost/timolol (n=126), latanoprost/timolol (n=97), timolol/dorzolamide (n=113), and brinzolamide/timolol (n=77).3
Mean IOP at baseline and 12 weeks after switching from latanoprost or travoprost monotherapy to GANFORT® UD3
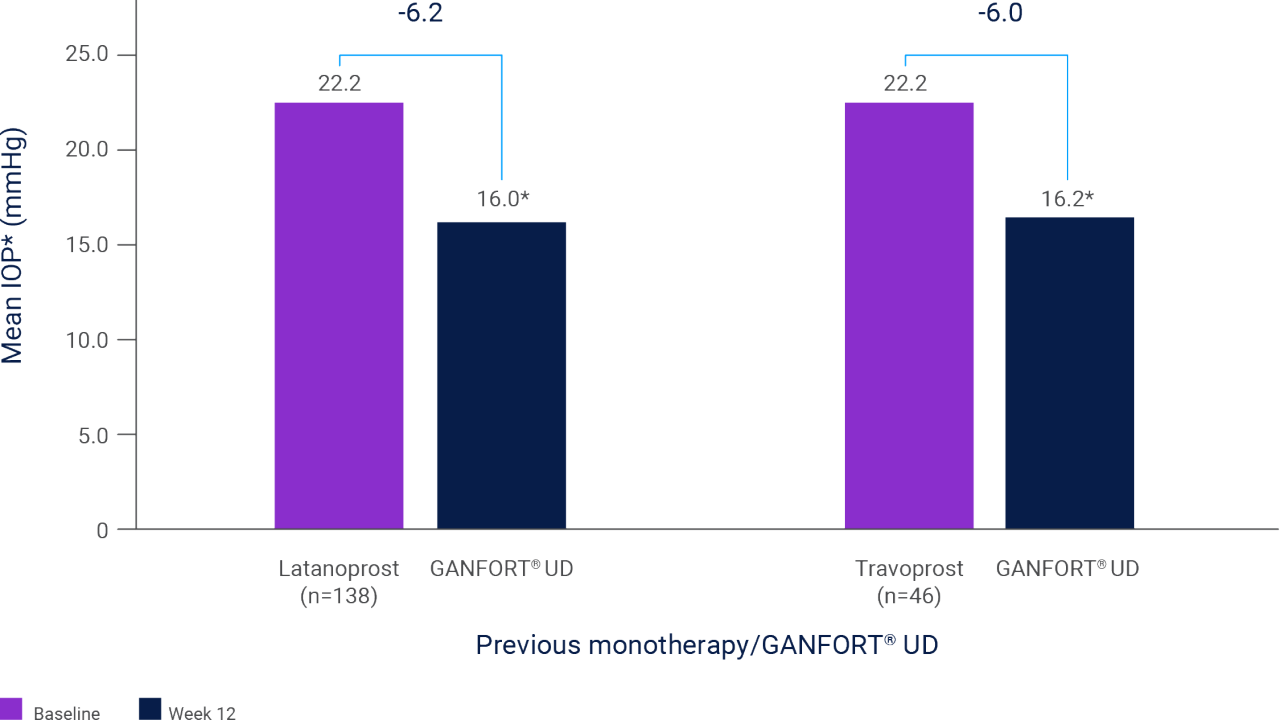
Adapted from Pfenningsdorf S et al. 2016.3
*p<0.0001 vs. baseline.
IOP, intraocular pressure; OHT, ocular hypertension; PF, preservative free; POAG, primary open-angle glaucoma; UD, unit dose.
Results of a prospective, observational open-label study which patients who switched to GANFORT® UD due to insufficient IOP control on prior therapies or for other reasons as well as treatment-naïve patients. IOP was measured at baseline and at ~12 weeks. Previous therapies reported in ≥5% of patients (per-protocol population) included latanoprost (n=206), PF latanoprost (n=71), bimatoprost (n=135), PF tafluprost (n=133), travoprost (n=123), timolol (n=245), brinzolamide (n=136), travoprost/timolol (n=126), latanoprost/timolol (n=97), timolol/dorzolamide (n=113), and brinzolamide/timolol (n=77).3
If monotherapy is well tolerated and effective, but has not lowered IOP to the target pressure, an additional drug of a different class should be considered,4 and evidence suggests that fixed-combination therapy, like GANFORT® (MD or UD formulations), is preferable to two separate medications5,6
Absolute mean change in IOP of eight fixed-combination therapies (95% CI) from a 2013 poster. Based on mixed treatment comparison methodology showing indirect comparisons.5
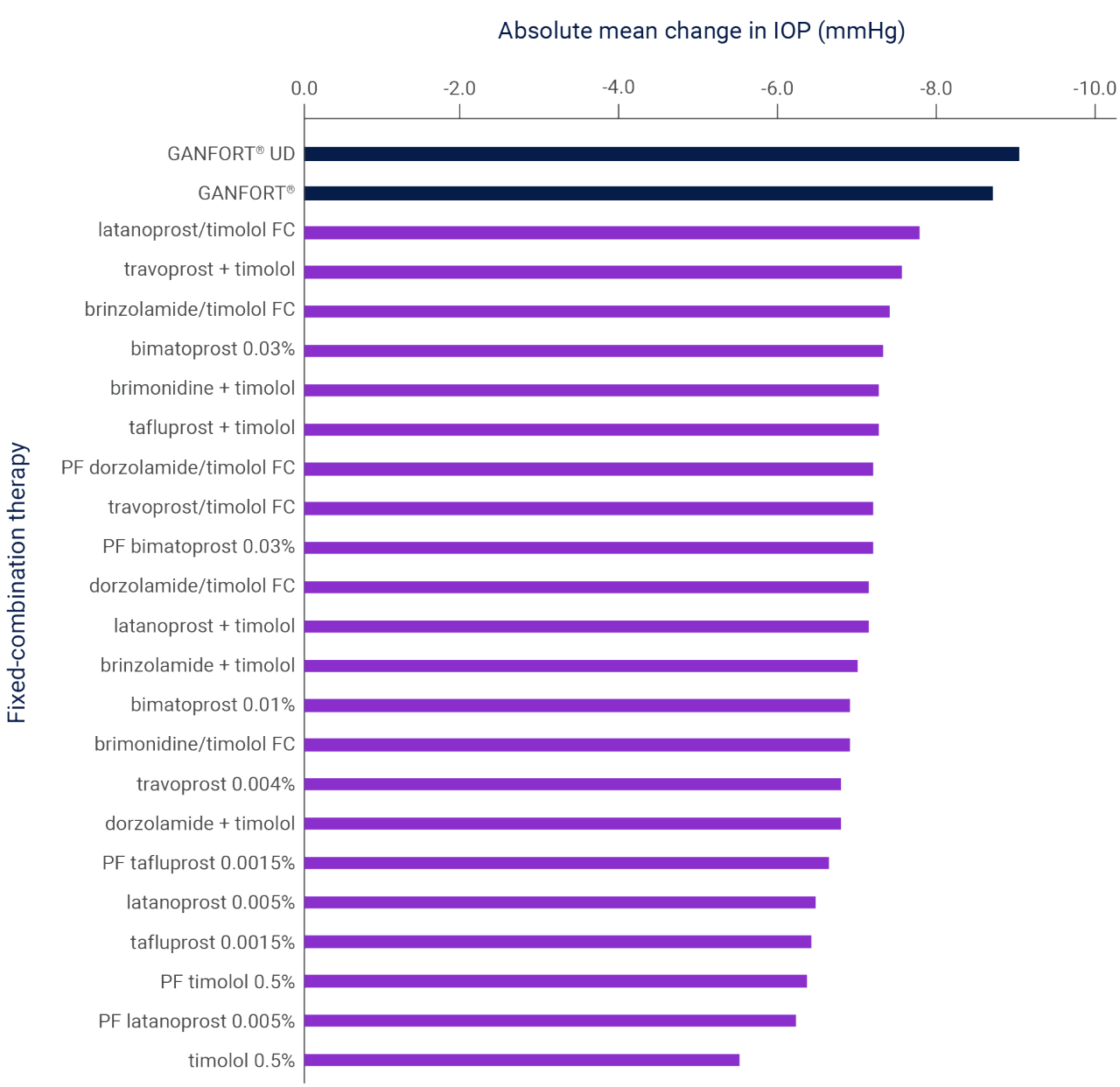
Adapted from Harvey B et al. 2013.5
CI, confidence interval; FC, fixed combination; IOP, intraocular pressure; OHT, ocular hypertension; PF, preservative free; POAG, primary open-angle glaucoma; UD, unit dose.
Results of a systematic literature review of 136 randomized controlled trials investigating the efficacy of combination therapies (both fixed and unfixed) for the treatment of POAG and OHT.5
Please refer to GANFORT® UD Summary of Product Characteristics for further information on adverse events.
GANFORT® single-dose is indicated for the reduction of intraocular pressure (IOP) in patients with open-angle glaucoma or ocular hypertension who are insufficiently responsive to topical beta-blockers or prostaglandin analogs.1
The majority of adverse reactions reported with GANFORT® single-dose were ocular, mild in severity and none were serious. Based on a 12-week study of GANFORT® single-dose administered once daily, the most commonly reported adverse reaction with GANFORT® single-dose was conjunctival hyperaemia (mostly trace to mild and thought to be of a non-inflammatory nature) in approximately 21% of patients and led to discontinuation in 1.4% of patients.1
Please refer to GANFORT® UD Summary of Product Characteristics for further information on adverse events.


