GANFORT® (bimatoprost/timolol ophthalmic solution)
GANFORT® is indicated for the reduction of intraocular pressure in adult patients with open-angle glaucoma or ocular hypertension who are insufficiently responsive to topical beta-blockers or prostaglandin analogs.1
GANFORT® UD (bimatoprost/timolol ophthalmic solution 0.03/0.5%)
GANFORT® could offer greater IOP reduction than its individual components used alone1
533 patients treated with GANFORT® benefitted from a significant reduction in IOP compared to its individual components of bimatoprost and timolol.2
Percentage of patients achieving >20% reduction in mean diurnal IOP from baseline at week 122
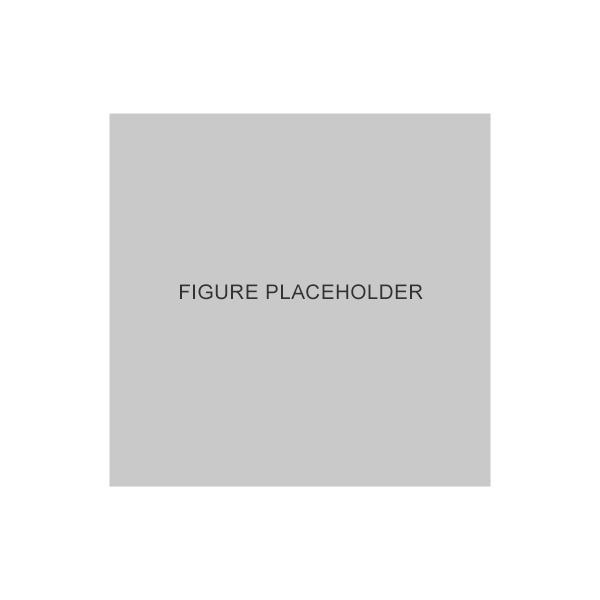
Adapted from Brandt JD et al. 2008.2
* p<0.001 vs. bimatoprost and vs. timolol.
IOP, intraocular pressure.
Results of two, identical, double-masked, parallel studies at 59 sites in the United States and Canada examining the efficacy and safety of GANFORT® taken once-daily in the morning vs. the individual components, bimatoprost 0.03% once in the evening and timolol 0.5% taken twice daily, over 3 months.2
If monotherapy is well tolerated and effective, but has not lowered IOP to the target pressure, an additional drug of a different class should be considered,3 and evidence suggests that fixed-combination therapy, like GANFORT® (MD or UD formulations), is preferable to two separate medications2,4
[The below figure and use of the reference in the callout above is market dependent if local markets can use posters]
Absolute mean change in IOP of eight fixed-combination therapies (95% CI) from a 2013 poster. Based on mixed treatment comparison methodology showing indirect comparisons.4
Adapted from Harvey B et al. 2013.4
CI, confidence interval; FC, fixed combination; IOP, intraocular pressure; OHT, ocular hypertension; PF, preservative free; POAG, primary open-angle glaucoma; UD, unit dose.
Results of a systematic literature review of 136 randomized controlled trials investigating the efficacy of combination therapies (both fixed and unfixed) for the treatment of POAG and OHT.4
GANFORT® was shown to lower IOP in 5,556 patients from five open-label studies who switched from prior monotherapy**,5
GANFORT® was shown to lower IOP in 5,556 patients from five open-label studies who switched from prior monotherapy**,5
** Results of five multicenter, observational, non-controlled, open-label studies throughout Europe. Patients were identified from 830 sites in Austria, France, Germany, The Netherlands, and Switzerland. Assessments were made at baseline, 6 weeks (in Austrian, German and Swiss centers), and 12 weeks in all centers, patient data were combined (N=5,556).5 Patients stepping up to GANFORT® from either latanoprost or travoprost monotherapy experienced a statistically significant reduction in IOP, p<0.0001 vs. baseline.5 The majority of patients reported no adverse events (90.3%). The most common AEs were conjunctival hyperaemia (3.2%) and eye irritation (2.8%).5
Mean IOP at baseline and 12 weeks after switching from prior monotherapy to GANFORT®5
Adapted from Pfenningsdorf S et al. 2013.5
* Bars represent the mean of right and left eye mean IOP differences from baseline.
** p<0.0001, baseline vs. final visit.
AE, adverse event; IOP, intraocular pressure; OHT, ocular hypertension; POAG, primary open-angle glaucoma.
Results of an analysis of five multicenter, observational, non-controlled, open-label, 12-week studies evaluating the efficacy and safety of GANFORT® in patients with POAG or OHT conducted at 830 sites in five countries, N=5,556.5 The majority of patients reported no adverse events (90.3%). The most common AEs were conjunctival hyperaemia (3.2%) and eye irritation (2.8%).5
GANFORT® is indicated for the reduction of intraocular pressure (IOP) in patients with open-angle glaucoma or ocular hypertension who are insufficiently responsive to topical beta-blockers or prostaglandin analogs.1
The majority of adverse reactions reported in clinical studies using GANFORT® were ocular, mild in severity and none were serious. Based on 12-month clinical data, the most commonly reported adverse reaction was conjunctival hyperaemia (mostly trace to mild and thought to be of a non-inflammatory nature) in approximately 26% of patients and led to discontinuation in 1.5% of patients.1
Please refer to GANFORT® Summary of Product Characteristics for further information on adverse events.
IOP, intraocular pressure.
1. GANFORT® (bimatoprost/timolol ophthalmic solution 0.03%/0.5%). Summary of Product Characteristics. 2022
2. Brandt JD et al. J Glaucoma 2008; 17: 211—216.
3. European Glaucoma Society (EGS). Terminology and Guidelines for Glaucoma. Fifth edition. EGS. 2020.
4. Harvey B et al. Poster presented at the World Congress on Controversies in Ophthalmology (COPHY). 4-7th April 2013. Budapest, Hungary.
5. Pfenningsdorf S et al. Clin Ophthalmol 2013; 7: 1219—1225.
05/2024 | ALL-LUM-240002


