GANFORT® (bimatoprost/timolol ophthalmic solution)
GANFORT® UD is indicated for the reduction of intraocular pressure in adult patients with open-angle glaucoma or ocular hypertension who are insufficiently responsive to topical beta-blockers or prostaglandin analogs.1
GANFORT® UD (bimatoprost/timolol ophthalmic solution 0.03/0.5%)
GANFORT® UD is a preservative-free, unit dose, fixed-combination therapy of two active substances: bimatoprost, a synthetic prostamide, and timolol, a β-blocker1
GANFORT® UD offers a dual mechanism of action1
Bimatoprost and timolol, the two active substances in GANFORT® UD, decrease elevated intraocular pressure by complementary mechanisms of action, and the combined effect results in additional IOP reduction compared to either compound administered alone. GANFORT® UD also has a rapid onset of action.1
GANFORT® UD delivers two complementary IOP-lowering modes of actions with the convenience of a single fixed dose1
Bimatoprost1,2
Timolol1,3
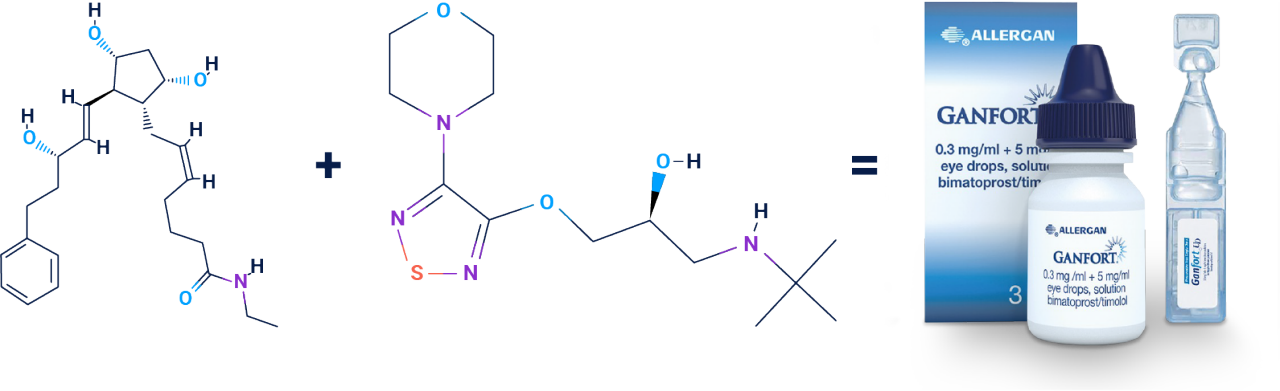
Bimatoprost1
Stimulates aqueous humor outflow via the trabecular meshwork and uveoscleral pathways1
Timolol1
Possibly reduce aqueous humor formation. The precise mode of action of timolol is unknown1
Adapted from GANFORT® UD Summary of Product Characteristics 2022, NCBI PubChem Bimatoprost Compound Summary, and NCBI PubChem Timolol Compound Summary.1-3
Administration and dosing for GANFORT® UD
GANFORT® UD combines bimatoprost 0.03% and timolol 0.5% in a single-dose, preservative-free container for one drop a day.1
Since multiple topical treatments may reduce adherence and increase exposure to preservatives, preservative-free fixed combination therapy such as GANFORT® UD is preferable to two separate instillations of agents.4
Patients that may benefit from preservative-free formulations like GANFORT® UD include patients who are likely to need long-term treatment, those who are allergic to preservatives, and those with clinically significant OSD.5
Potential benefits of preservative free formulations may include:6
GANFORT® UD is indicated for the reduction of intraocular pressure (IOP) in patients with open-angle glaucoma or ocular hypertension who are insufficiently responsive to topical beta-blockers or prostaglandin analogs.1
GANFORT® UD may not be suitable for some of your patients
GANFORT® UD has several contraindications:1
Please refer to GANFORT® UD Summary of Product Characteristics for full safety information and precautions for use.
GANFORT® single-dose is indicated for the reduction of intraocular pressure (IOP) in patients with open-angle glaucoma or ocular hypertension who are insufficiently responsive to topical beta-blockers or prostaglandin analogs.1
The majority of adverse reactions reported with GANFORT® single-dose were ocular, mild in severity and none were serious. Based on a 12-week study of GANFORT® single-dose administered once daily, the most commonly reported adverse reaction with GANFORT® single-dose was conjunctival hyperaemia (mostly trace to mild and thought to be of a non-inflammatory nature) in approximately 21% of patients and led to discontinuation in 1.4% of patients.1
Please refer to GANFORT® UD Summary of Product Characteristics for further information on adverse events.
IOP, intraocular pressure; OSD, ocular surface disease; UD. unit dose.
1. GANFORT UD (bimatoprost/timolol ophthalmic solution 0.03%/0.5%). Summary of Product Characteristics. 2022.
2. Bimatoprost Compound Summary. National Library of Medicine. Available at: https://pubchem.ncbi.nlm.nih.gov/compound/Bimatoprost. Accessed May 2024.
3. Timolol Compound Summary. National Library of Medicine. Available at: https://pubchem.ncbi.nlm.nih.gov/compound/Timolol. Accessed May 2024.
4. European Glaucoma Society (EGS). Terminology and Guidelines for Glacuoma. Fifth edition. EGS. 2020.
5. Stalmans I et al. Eur J Ophthalmol 2013; 23(4): 518—525.
6. Thygesen J. Clin Ophthalmol 2018; 12: 707—717.
05/2024 | ALL-LUM-240002


