GANFORT® (bimatoprost/timolol ophthalmic solution)
GANFORT® is indicated for the reduction of intraocular pressure in adult patients with open-angle glaucoma or ocular hypertension who are insufficiently responsive to topical beta-blockers or prostaglandin analogs.1
GANFORT® UD (bimatoprost/timolol ophthalmic solution 0.03/0.5%)
GANFORT® is a fixed-combination therapy of two active substances: bimatoprost, a synthetic prostamide, and timolol, a β-blocker1
GANFORT® offers a dual mechanism of action1
Bimatoprost and timolol, the two active substances in GANFORT®, decrease elevated intraocular pressure by complementary mechanisms of action, and the combined effect results in additional IOP reduction compared to either compound administered alone. GANFORT® also has a rapid onset of action.1
GANFORT® delivers two complementary IOP-lowering modes of actions with the convenience of a single fixed dose1
Bimatoprost1,2
Timolol1,3
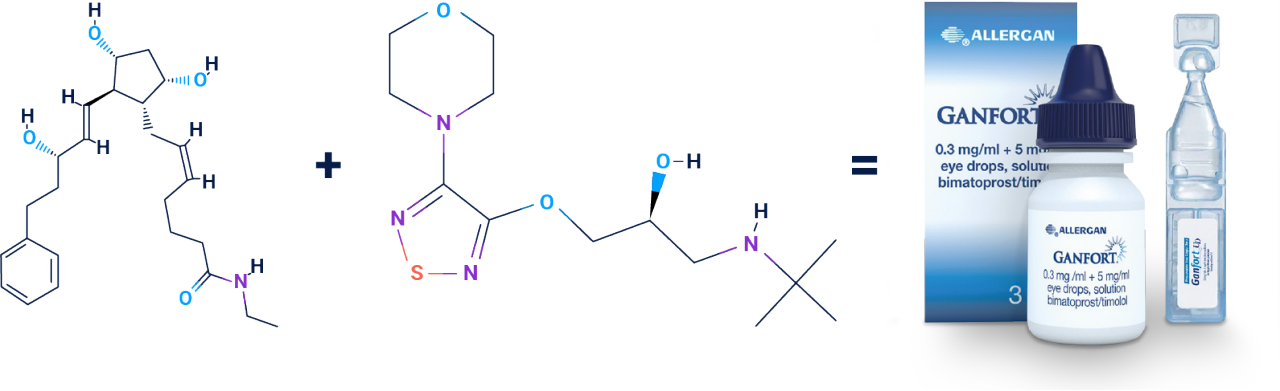
1 – Stimulates aqueous humor outflow via the trabecular meshwork and uveoscleral pathways1
2 – Possibly reduces aqueous humor formation. The precise mode of action of timolol is unknown1
Adapted from GANFORT® Summary of Product Characteristics 2022, NCBI PubChem Bimatoprost Compound Summary, and NCBI PubChem Timolol Compound Summary.1-3
Administration and dosing for GANFORT®
GANFORT® combines bimatoprost 0.03% and timolol 0.5% in a single bottle for one drop a day.1
Since multiple topical treatments may reduce adherence and increase exposure to preservatives, fixed combination therapy such as GANFORT® is preferable to two separate instillations of agents.4
GANFORT® is indicated for the reduction of intraocular pressure (IOP) in patients with open-angle glaucoma or ocular hypertension who are insufficiently responsive to topical beta-blockers or prostaglandin analogs.1
GANFORT® may not be suitable for some of your patients
GANFORT® has several contraindications:1
Please refer to GANFORT® Summary of Product Characteristics for full safety information and precautions for use.
GANFORT® is indicated for the reduction of intraocular pressure (IOP) in patients with open-angle glaucoma or ocular hypertension who are insufficiently responsive to topical beta-blockers or prostaglandin analogs.1
The majority of adverse reactions reported in clinical studies using GANFORT® were ocular, mild in severity and none were serious. Based on 12-month clinical data, the most commonly reported adverse reaction was conjunctival hyperaemia (mostly trace to mild and thought to be of a non-inflammatory nature) in approximately 26% of patients and led to discontinuation in 1.5% of patients.1
Please refer to GANFORT® Summary of Product Characteristics for further information on adverse events.
IOP, intraocular pressure.
1. GANFORT® (bimatoprost/timolol ophthalmic solution 0.03%/0.5%). Summary of Product Characteristics. 2022.
2. Bimatoprost Compound Summary. National Library of Medicine. Available at: https://pubchem.ncbi.nlm.nih.gov/compound/Timolol. Accessed May 2024.
3. Timolol Compound Summary. National Library of Medicine. Available at: https://pubchem.ncbi.nlm.nih.gov/compound/Timolol. Accessed May 2024.
4. European Glaucoma Society (EGS). Terminology and Guidelines for Glaucoma. Fifth edition. EGS. 2020.


