EXPEDITION
ITT: Intention-to-treat; HCV: hepatitis C virus
* Both patients reached the sustained virologic response rate at 12 weeks post-treatment
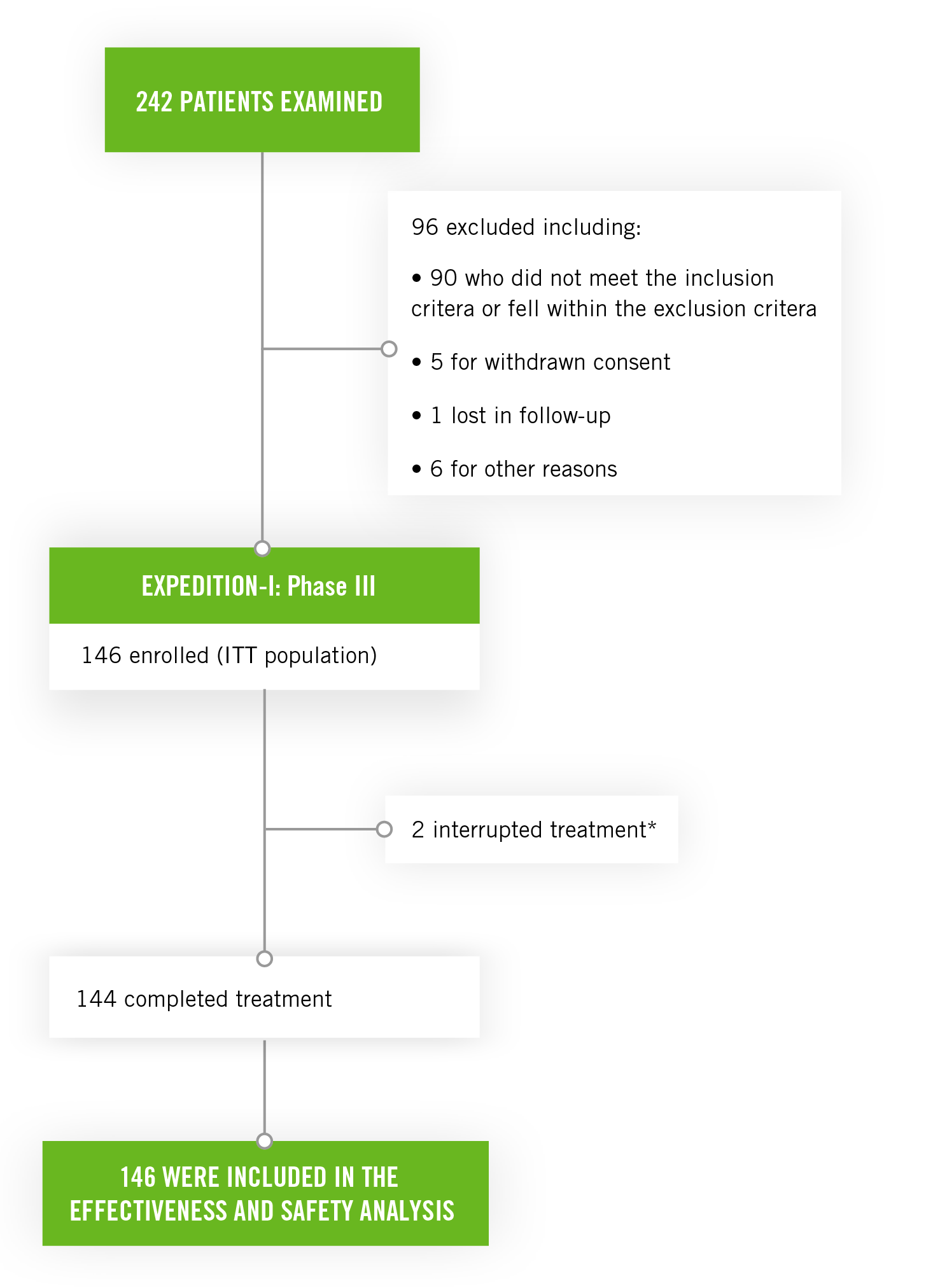
The study enrolled 146 patients with compensated cirrhosis including:
- 48 (33%) patients with genotype 1a HCV infection
- 39 (27%) patients with genotype 1b HCV infection
- 34 (23%) patients with genotype 2 HCV infection
- 16 (11%) patients with genotype 4 HCV infection
- 2 (1%) patients with genotype 5 HCV infection
- 7 (5%) patients with genotype 6 HCV infection
The majority of patients were white males and naïve to treatment. Of the 36 previously treated patients, 25 (69%) experienced virologic failure with the previous treatment containing interferon or pegylated interferon with or without ribavirin, while 11 (31%) had experienced virologic failure with a previous treatment containing sofosbuvir plus ribavirin with (n=2) or without (n=9) pegylated interferon.
HCV: hepatitis C virus; CI: confidence interval; ITT: intention-to-treat; SVR12: sustained virologic response at 12 weeks post-treatment
Results
The rate of SVR12 was demonstrated for each genotype and the total population.
The error bars (shown only for the groups with more than ten patients) are CI 95% of the Wilson score at 95% on two sides. The exact number of patients who reached SVR12 were 89 of 90 with genotype 1 HCV infection, 31 of 31 with genotype 2 infection, 16 of 16 with genotype 4 infection, 2 of 2 with genotype 5, 7 of 7 with genotype 6 and 145 of 146 patients total.
Safety results
The most common events (occurring in ≥ 10% of patients) were headache, fatigue, and pruritus. Serious AEs occurred in 11 patients (8%). No patients discontinued treatment due to AEs.
EXPEDITION-II: Phase III registration clinical study2
Study design
The EXPEDITION-2, open-label, multicenter, phase 3 study evaluated treatment with glecaprevir/pibrentasvir (300 mg/120 mg) in adult patients with co-infection from genotype 1-6 HCV/HIV-1 with or without compensated cirrhosis for 8 and 12 weeks respectively.
Results
The single administration of glecaprevir in combination with pibrentasvir (glecaprevir/pibrentasvir) demonstrated elevated rates of sustained virologic response at 12 weeks after treatment (SVR12) in patients with genotype 1-6 hepatitis C virus infection (HCV) and co-infection from HIV-1, including patients with compensated cirrhosis.
Safety results
The most common events (occurring in ≥5% of patients) were headache, fatigue, nausea, and nasopharyngitis. Serious AEs occurred in 4 patients (1%). One patient discontinued treatment due to AEs.
Conclusions
Glecaprevir/pibrentasvir, administered for eight weeks in non-cirrhotic patients and for 12 weeks in cirrhotic patients, demonstrated efficacy and was a treatment for HCV/HICV-1 [sic: HIV-1] co-infection, independent of the viral load of HCV or previous treatment with interferon or sofosbuvir. The rates of SVR achieved with glecaprevir/pibrentasvir in patients co-infected by HCV/HIV-1, were similar to those obtained in patients with a HCV infection alone. One patient discontinued treatment due to adverse events.
AE: adverse event; HCV: hepatitis C virus; HIV: human immunodeficiency virus; SVR12: Sustained virologic response at 12 weeks post-treatment.
EXPEDITION-IV: Phase III registration clinical study3
Background
Chronic hepatitis (from the hepatitis C virus) occurs more frequently in patients with chronic renal disease than those without. Patients with chronic renal disease with HCV infection are more at risk of progression towards the terminal stage of renal disease than those with chronic renal disease but no HCV infection. Patients with chronic renal disease associated with HCV infection have limited treatment options.
Results
Of the 104 patients enrolled in the study, 52% had a genotype 1 HCV infection, 16% had genotype 2, 11% genotype 3, 19% genotype 4 and 2% genotype 5 or 6. The rate of sustained virologic response was 98% (102 of 104 patients; CI 95%, 95 to 100).
Safety results
The most common events (occurring in ≥10% of patients) were headache, fatigue, and diarrhea. Serious AEs occurred in 5 patients (1%). No patients discontinued treatment due to AEs.
Conclusions
Treatment with glecaprevir and pibrentasvir for 12 weeks achieved an elevated rate of sustained virologic response in patients with stage 4 or 5 chronic renal disease and HCV infection.
Treatment without ribavirin, a combination with glecaprevir and pibrentasvir, led to an elevated virologic response rate; no patients experienced virologic failure, independent of the HCV genotype, presence or absence of cirrhosis or other baseline factors.
AE: adverse event; HCV: hepatitis C virus; CI: confidence interval
EXPEDITION-8: Phase III registration clinical study4
Study design
EXPEDITION-8 was a single-arm, open-label, phase IIIb study in GT 1–6 treatment-naïve, compensated cirrhotic patients (N=343) who received MAVIRET for 8 weeks.
Results
The SVR12 rate in patients with GT 1–6 was 99.7% (334/335; 95% CI, 98.3–99.9) in the PP population and 97.7% (335/343; 95% CI, 96.1–99.3) in the ITT population.
Safety results
The most common events (occurring in ≥5% of patients) were headache, fatigue, nausea, and pruritus. Serious AEs occurred in 6 patients (2%). No patients discontinued treatment due to AEs.
Conclusions
8-week glecaprevir/pibrentasvir was well tolerated, with a discontinuation rate due to AEs of 0% and led to a similarly high SVR12 rate as the 12-week regimen in treatment-naïve patients with chronic HCV GT1-6 infection and compensated cirrhosis.
I want to receive more information via a product specialist
References
- Forns X, Lee SS, Valdes J, et al. Glecaprevir plus pibrentasvir for chronic hepatitis C virus genotype 1, 2, 4, 5, or 6 infection in adults with compensated cirrhosis (EXPEDITION-1): a single-arm, open-label, multicentre phase 3 trial. Lancet Infect Dis. 2017;17(10):1062-1068. doi:10.1016/S1473-3099(17)30496-6
- Rockstroh J, Lacombe K, Viani RM, et al. Efficacy and safety of glecaprevir/pibrentasvir in patients coinfected with hepatitis C virus and human immunodeficiency virus-1: the EXPEDITION-2 study. Clin Infect Dis. 2018;67(7):1010-1017. doi:10.1093/cid/ciy220
- Gane E, Lawitz E, Pugatch D, et al. Glecaprevir and pibrentasvir in patients with HCV and severe renal impairment. N Engl J Med. 2017;377(15):1448-1455. doi:10.1056/NEJMoa1704053
- Brown RS, Buti M, Rodrigues L, et al. Glecaprevir/pibrentasvir for 8 weeks in treatment-naïve patients with chronic HCV genotypes 1–6 and compensated cirrhosis: the EXPEDITION-8 trial [published online November 1, 2019]. J Hepatol. doi:10.1016/j.jhep.2019.10.020.
Adverse events should be reported. Reporting forms and information can be found at yellowcard.mhra.gov.uk.
Adverse events should also be reported to AbbVie on uk_pvvendor@abbvie.com