ENDURANCE
ENDURANCE-1 and -3: Phase III registration clinical studies
HCV: hepatitis C virus; SVR12: sustained virologic response at 12 weeks post-treatment
Safety results
The most common events across both trials (occurring in ≥ 10% of patients) were headache, fatigue, and nausea. Serious adverse events occurred in 9 patients (1%) in ENDURANCE-1 and 10 patients (2%) in ENDURANCE-3. Two patients prematurely discontinued treatment due to AEs in both trials. Three additional patients discontinued due to AEs in ENDURANCE-3.
Study summary
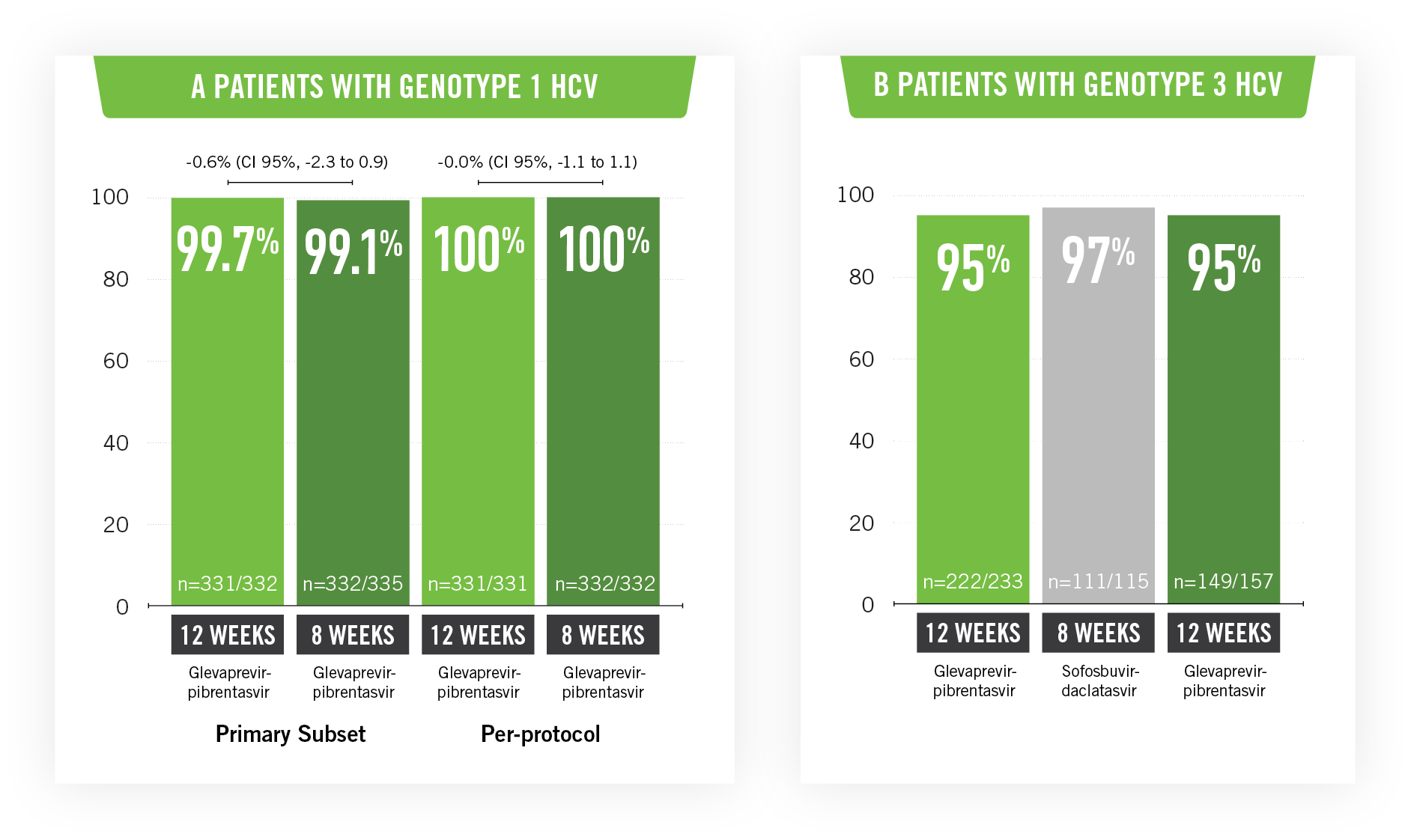
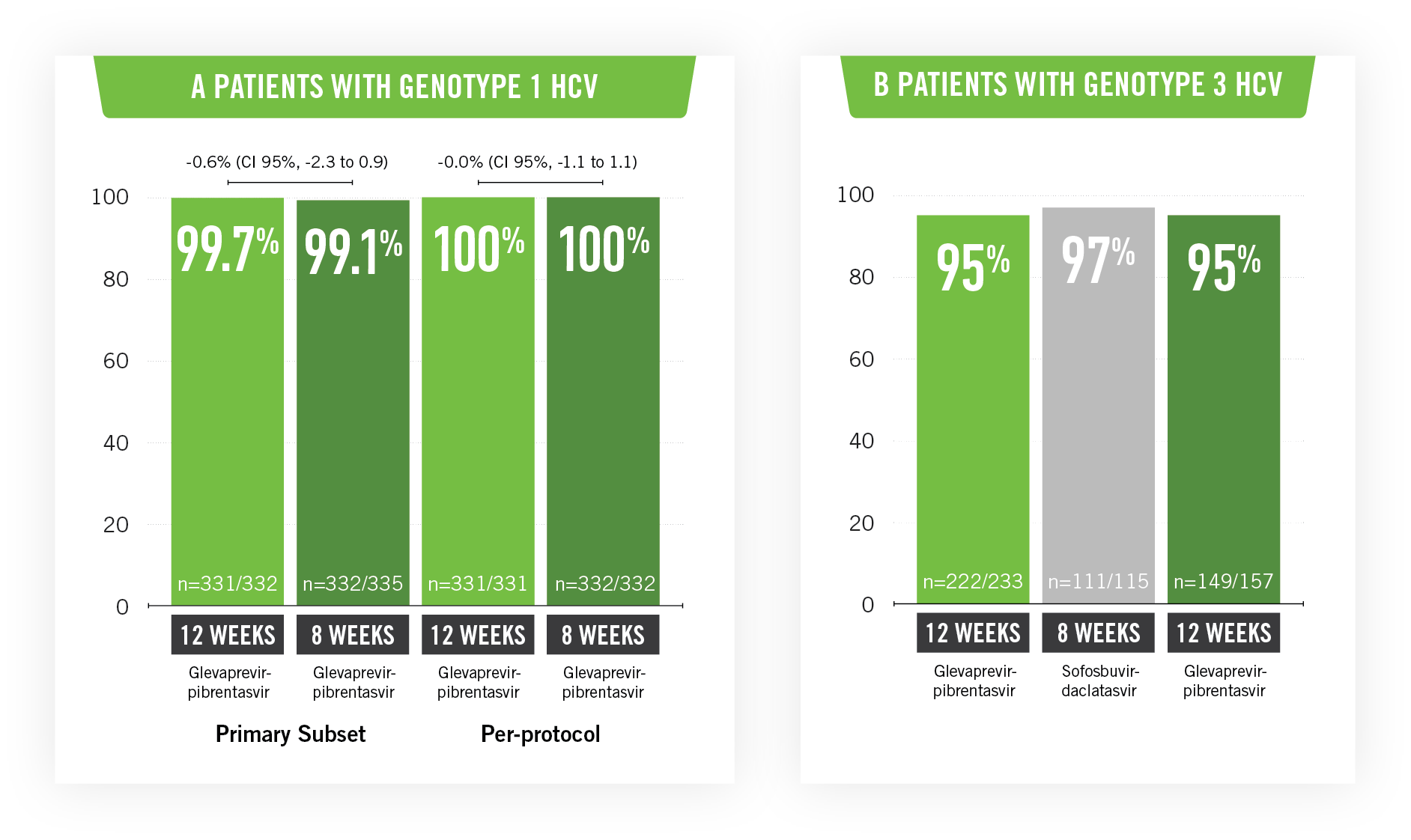
In total, 1,280 patients were treated. The sustained virologic response rate at 12 weeks in patients with genotype 1 HCV infection was 99.1% (CI 95%, 98 to 100) in the 8-week group and 99.7% in the 12-week group.
Patients with genotype 3 HCV infection who were treated for 12 weeks with glecaprevir-pibrentasvir achieved a response rate at 12 weeks of 95% (CI 95%, 93 to 98; 222 of the 233 patients) and 97% (CI 95%; 93 to 99.9; 111 of 115) for those on treatment with sofosbuvir-daclatasvir; treatment with glecaprevir-pibrentasvir for eight weeks demonstrated a rate of 95% (CI 95%; 91 to 98; 149 of 157 patients).
Treatment with glecaprevir-pibrentasvir in a single administration for 8 or 12 weeks achieved elevated rates of sustained virologic response in patients with genotype 1 or 3 HCV infection who did not have cirrhosis.
I want to receive more information via a product specialist
Reference
- Zeuzem S., Foster G.R. et al. Glecaprevir–Pibrentasvir for 8 or 12 Weeks in HCV Genotype 1 or 3 Infection. N Engl J Med 2018;378:354-69. DOI: 10.1056/NEJMoa1702417
Adverse events should be reported. Reporting forms and information can be found at yellowcard.mhra.gov.uk.
Adverse events should also be reported to AbbVie on uk_pvvendor@abbvie.com