SURVEYOR
SURVEYOR-II Part 3: Phase III registration clinical study2
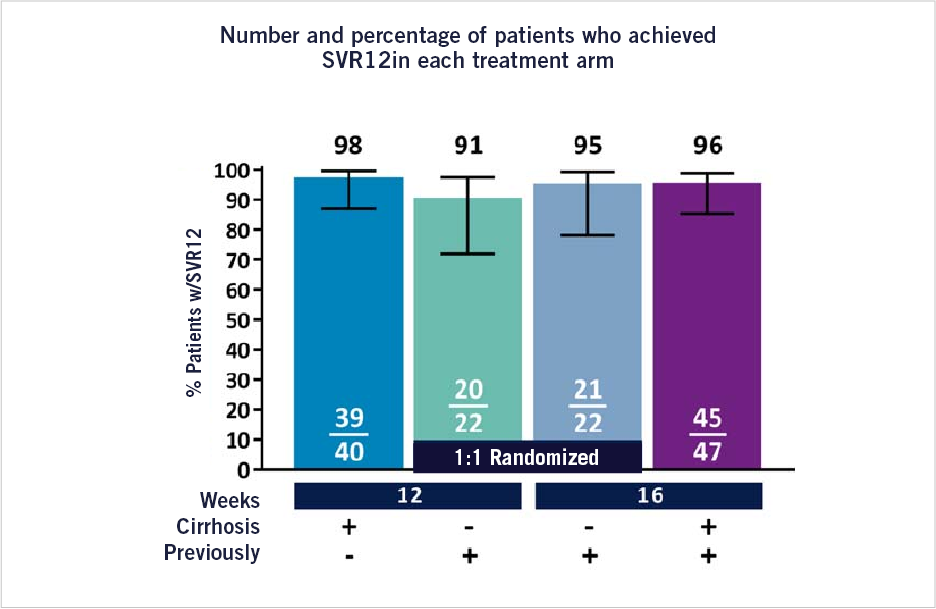
SURVEYOR-II Part 3 is a partially randomized, multicenter phase 3 study that evaluated the effectiveness and safety of glecaprevir/pibrentasvir (G/P) in adult patients with chronic infection from the genotype 3 hepatitis C virus, including patients with compensated cirrhosis and/or those previously treated for HCV. In total 189 patients were analyzed and enrolled in the study, 132 patients with genotype 3 HCV infection, and 131 began treatment.
Study design
The non-cirrhotic previously treated patients were randomized 1:1 to receive treatment with GLE - developed by Abbvie and Enanta - for 12 or 16 weeks and co-formulated with PIB (G/P). Treatment-naïve patients or those with compensated cirrhosis who were previously treated received G/P for 12 or 16 weeks, respectively. All patients received 3 tablets containing 100 mg/40 mg once a day by mouth, for a total dose of 300 mg/120 mg.
Treatment-naïve patients with compensated cirrhosis were treated with G/P for 12 weeks; patients with compensated cirrhosis who had been previously treated were treated for 16 weeks.
HCV: hepatitis C virus; CI: confidence interval; GLE (G): glecaprevir; PIB (P): pibrentasvir; SVR12: virologic response at 12 weeks post-treatment
Safety results
The majority of AEs were classified as mild, and no patients prematurely discontinued study drug due to AEs. Fatigue and headache were the only AEs reported in >10% of total patients across both treatment arms, at 22% and 19%, respectively. Six serious AEs were reported; none were attributed to study drugs by investigators, and none of the patients discontinued study drug. The type and frequency of AEs appeared similar between 12- and 16-week durations of G/P treatment. No hepatic decompensation events were observed. No patients experienced clinically significant ALT elevations.
Conclusions
Overall, the efficacy results from this study demonstrate that glecaprevir/pibrentasvir by mouth is a direct antiviral treatment with an elevated cure rate that does not require the co-administration of ribavirin in patients with genotype 3 HCV infection, independent of other typical negative predictive factors of response such as previous treatment experience or compensated cirrhosis.
SUMMARY
- The SURVEYOR-II study, Part 3 enrolled and treated several of the patients with HCV infection who were most difficult to treat: those with genotype 3 and/or cirrhotic previously treated patients.
- Overall, the fixed-dose combination of glecaprevir/pibrentasvir without ribavirin in a single administration was well tolerated with a discontinuation rate due to AEs of 0% and demonstrated an elevated rate of SVR12 (95%) in cirrhotic treatment-naïve patients treated for 12 weeks and in cirrhotic and non-cirrhotic previously treated patients treated for 16 weeks.
- Glecaprevir/pibrentasvir thus provides a treatment option in a single administration without ribavirin, which is effective and well tolerated in patients with genotype 3 HCV infection and/or previously treated cirrhotic patients.
- The most common events (occurring in ≥10% of patients) were headache and fatigue. Serious AEs occurred in 1 patient (6%). No patients discontinued treatment due to AEs.
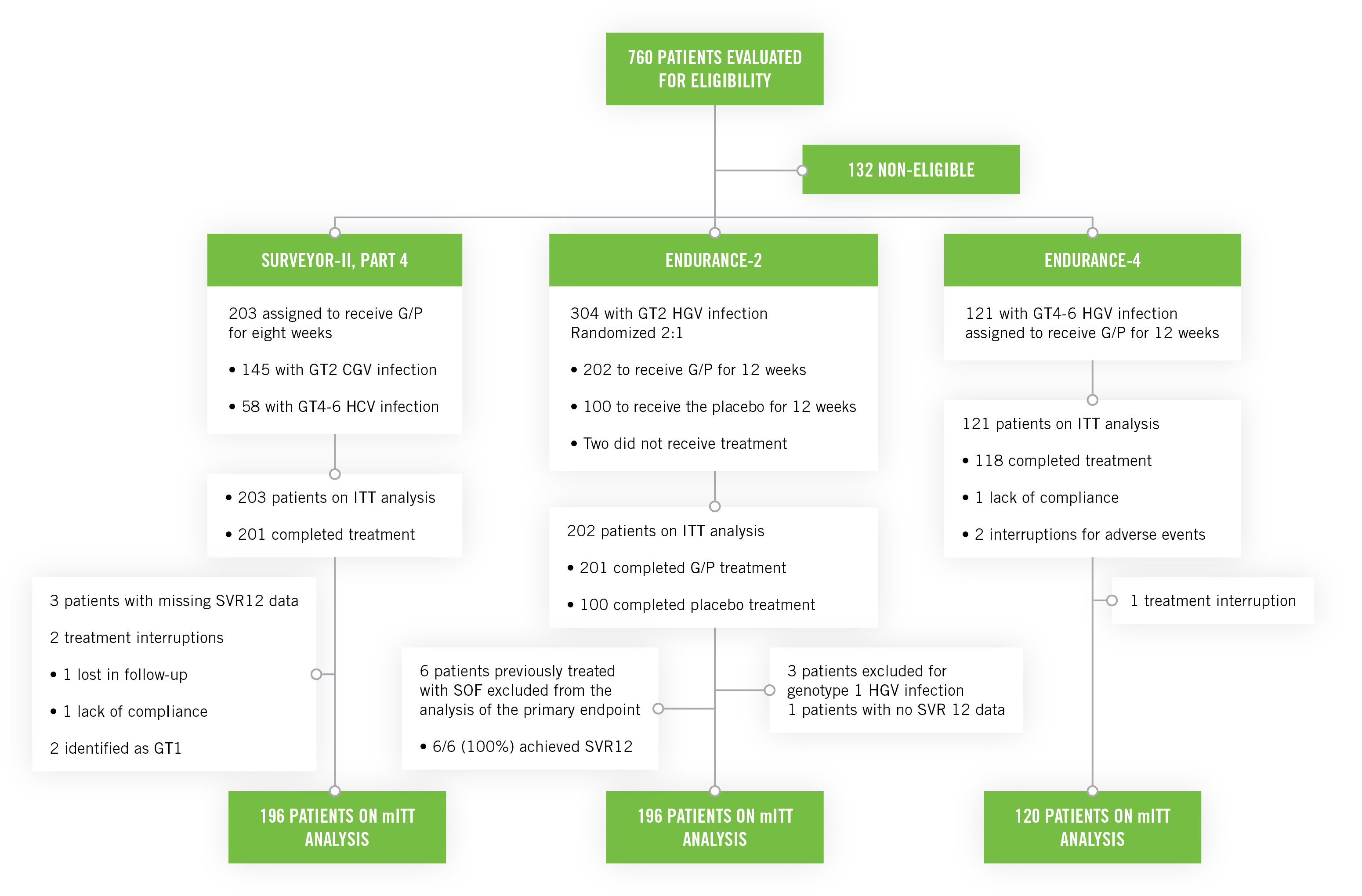
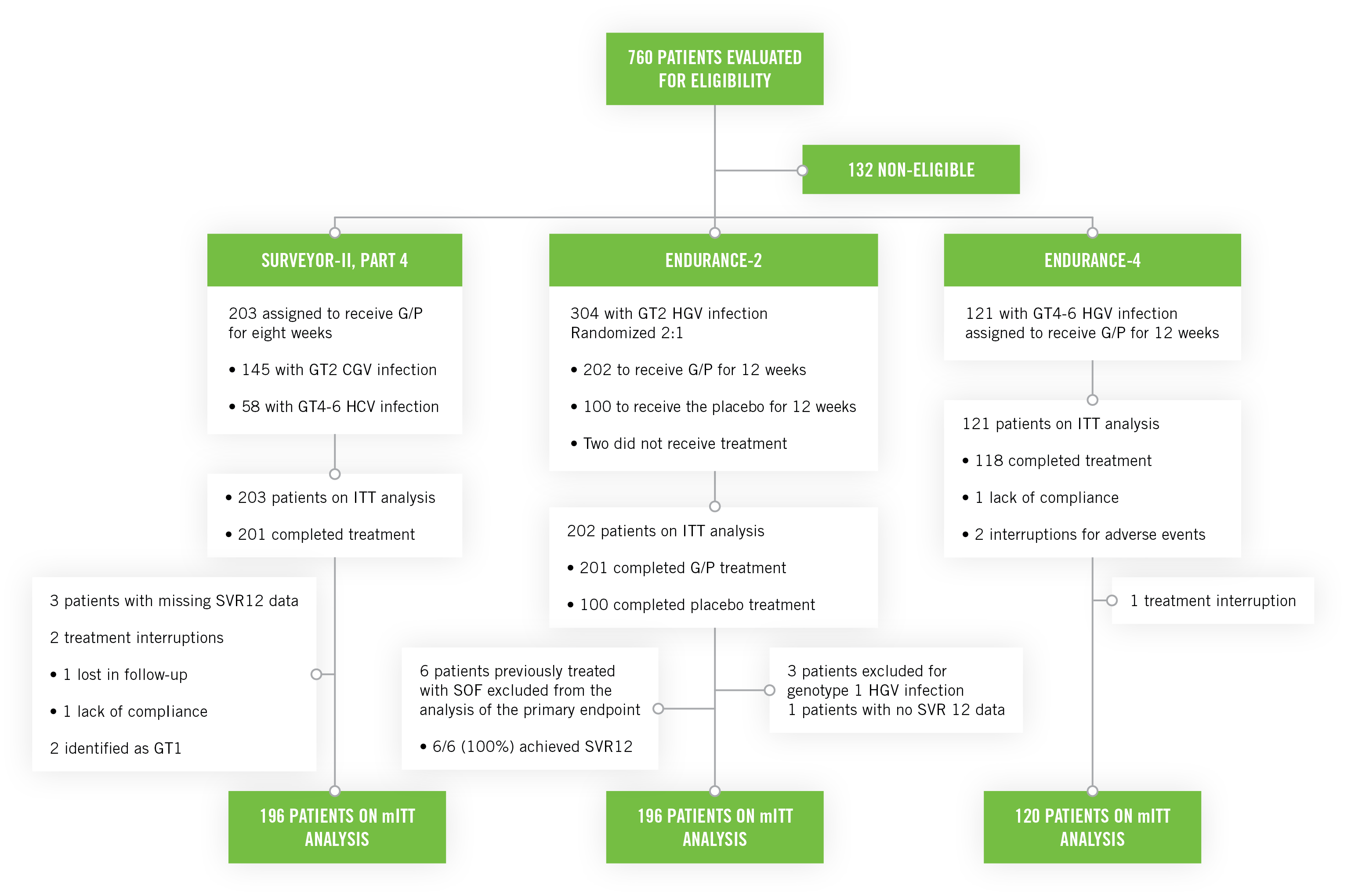
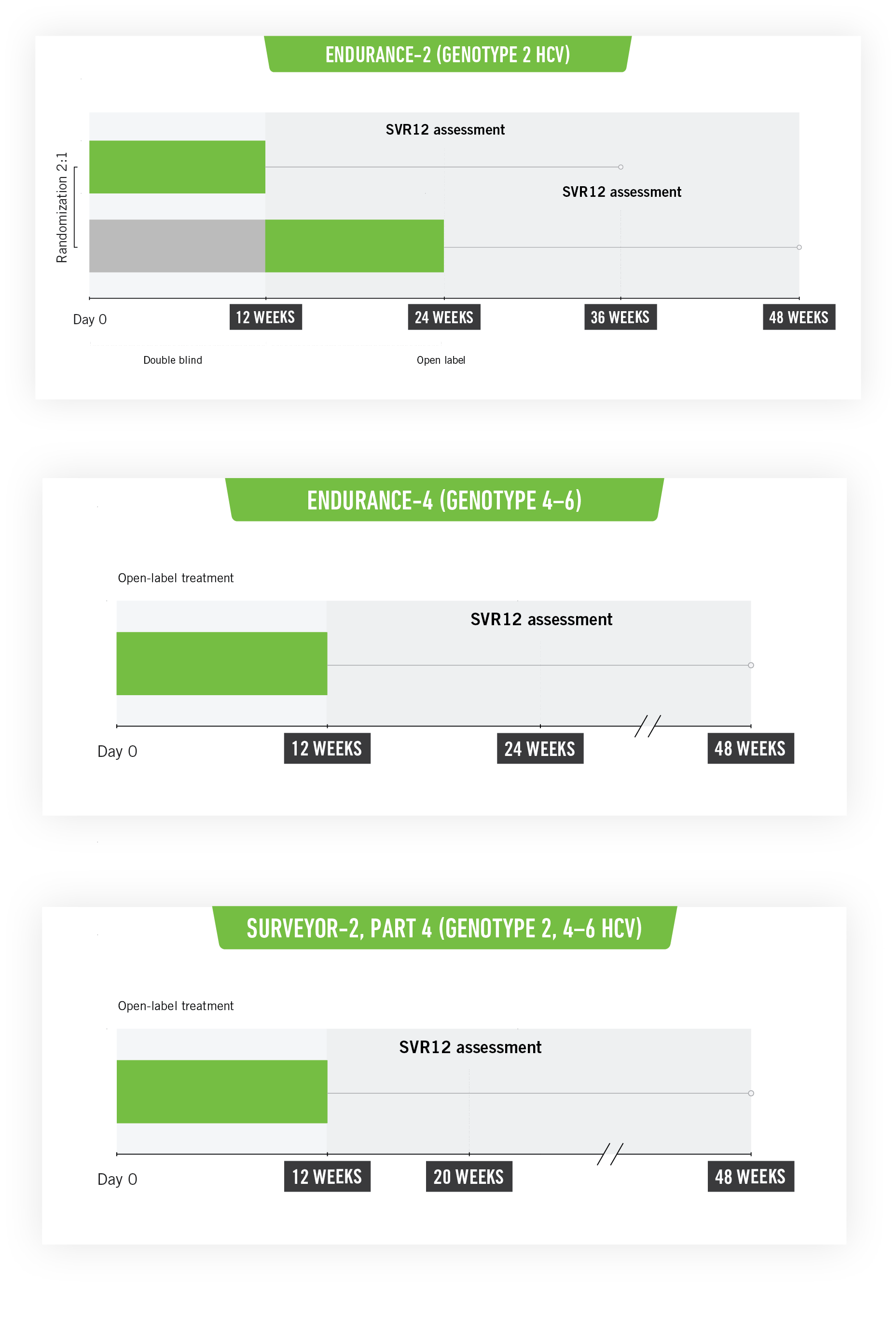
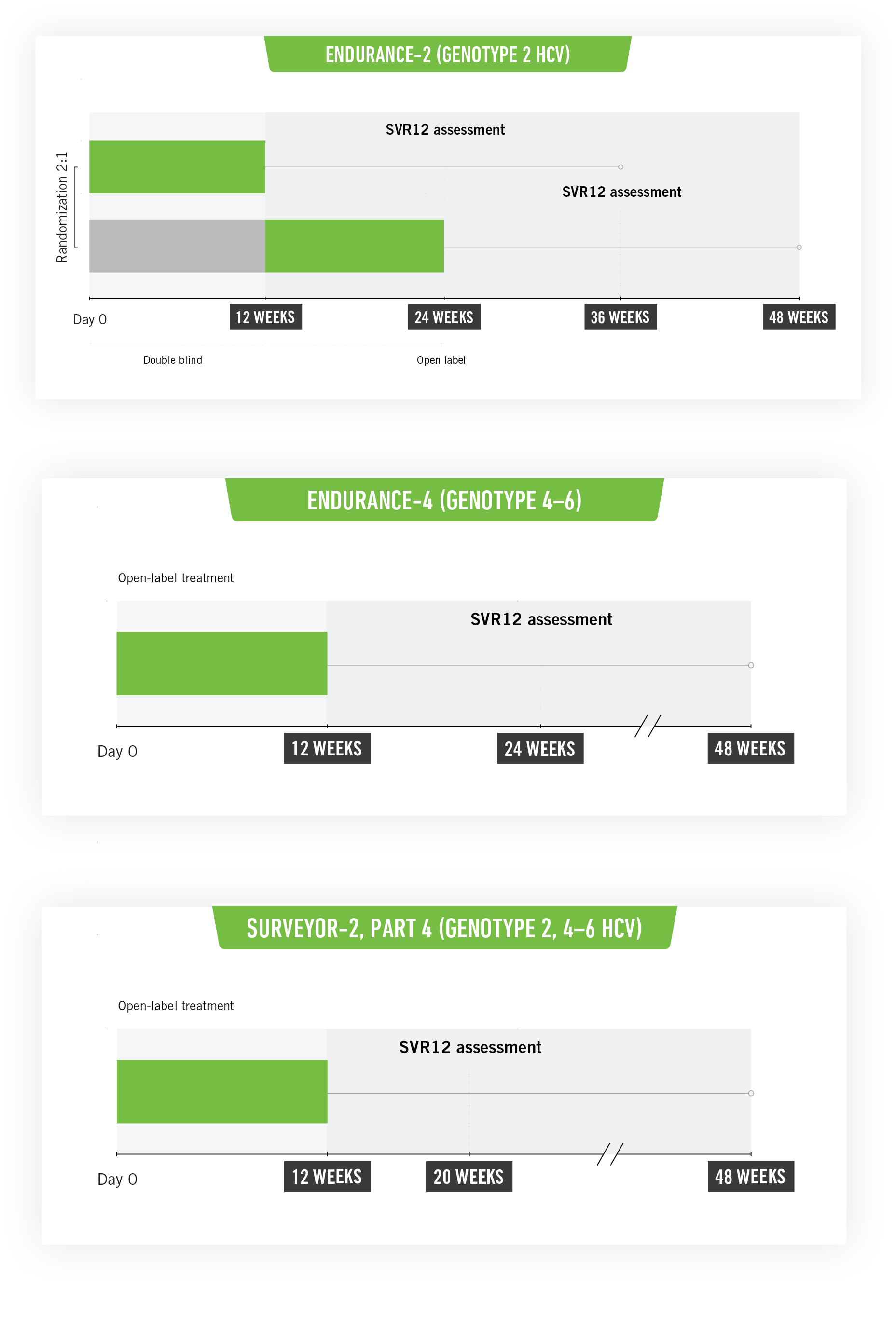
SURVEYOR-II Part 4, ENDURANCE-4, ENDURANCE-2: Phase III registration clinical studies3
SURVEYOR-II Part 4 and ENDURANCE-4
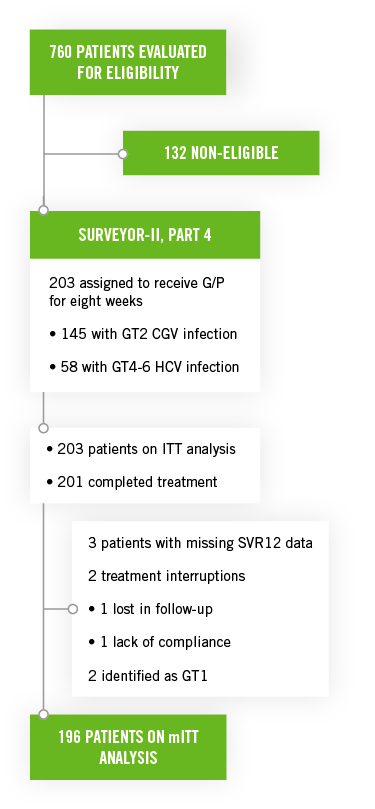
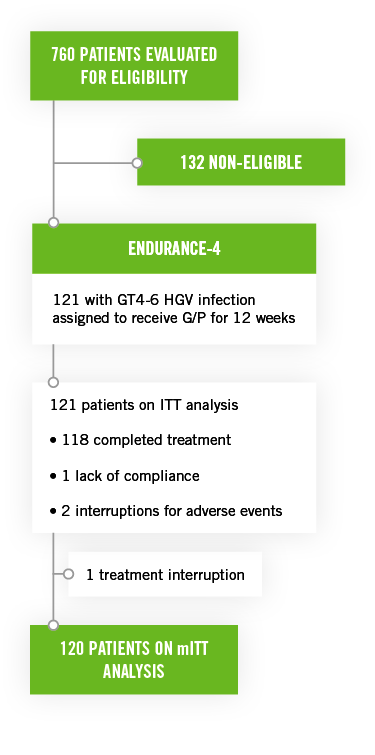
These studies included untreated or previously treated non-cirrhotic adult patients with genotype 2, genotype 4, genotype 5, or genotype 6 HCV infection, who received glecaprevir/pibrentasvir once a day by mouth in ENDURANCE-4 (n=121) and in SURVEYOR-II (n=145) for 12 or 8 weeks, respectively.
ENDURANCE-2
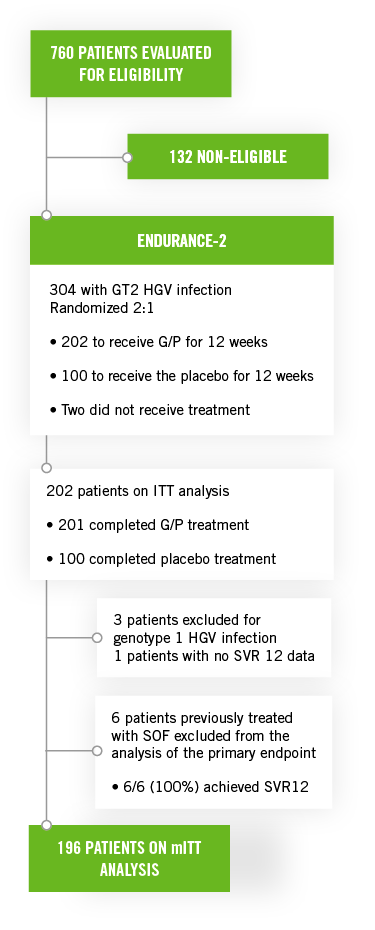
This study included untreated or previously treated non-cirrhotic adult patients with genotype 2 HCV infection. Patients were assigned randomly (2:1) to groups that received glecaprevir/pibrentasvir once a day by mouth (n=202; 300 mg/120 mg) or placebo (n=101) for 12 weeks.
Primary endpoint
Sustained virologic response at 12 weeks post treatment (SVR12) in the intention-to-treat (ITT) population.
Safety results
The most common events (occurring in ≥10% of patients) were headache and fatigue. Serious adverse events occurred in 7 patients (1%). Three patients discontinued treatment due to adverse events.
SUMMARY
ENDURANCE-2
- In patients with genotype 2 HCV infection, naïve to treatment with SOF, G/P for 12 weeks achieved a rate of SVR12 of 99%
- The six patients previously treated with SOF excluded from the primary analysis, all with SVR12 rate achieved, had an overall SVR12 of 99.5%
- The most common events (occurring in ≥10% of patients) were headache, fatigue, and nausea. Serious adverse events occurred in 3 patients (1%). No patients discontinued treatment due to AEs.
- The overall rate of SVR12 in patients with genotype 4 HCV infection (n=76), 5 (n=26) or 6 (n=19) treated for 12 weeks was 99%
- The most common events (occurring in ≥10% of patients) were headache, fatigue, and nausea. Serious adverse events occurred in 2 patients (2%). Three patients discontinued treatment due to AEs.
- The rate of SVR12 in patients with genotype 2 HCV infection treated for eight weeks was 98%.
- Five of the six patients previously treated with SOF for eight weeks achieved the rate of SVR12.
- The most common events (occurring in ≥10% of patients) were headache, fatigue, and nausea. Serious adverse events occurred in 1 patient (1%). No patients discontinued treatment due to AEs.
I want to receive more information via a product specialist
References
- Kwo P.Y, Poordad F. et al. Glecaprevir and pibrentasvir yield high response rates in patients with HCV genotype 1-6 without cirrhosis. Journal of Hepatology 2017 vol. 67, 263-271. doi: http://dx.doi.org/10.1016/j.jhep.2017.03.039
- Wyles D., Poordad F. et al. Glecaprevir/Pibrentasvir for Hepatitis C Virus Genotype 3 Patients With Cirrhosis and/or Prior Treatment Experience: A Partially Randomized Phase 3 Clinical Trial. Hepatology. Vol. 67, No.2, 201 doi: doi/10.1002/hep.29541
- Asselah T., Kowdley K.V., et al. Efficacy of Glecaprevir/Pibrentasvir for 8 or 12 Weeks in Patients with HCV Genotype 2, 4, 5, or 6 Infection Without Cirrhosis. Clinical Gastroenterology and Hepatology (2017). doi: 10.1016/j.cgh.2017.09.027.
Adverse events should be reported. Reporting forms and information can be found at yellowcard.mhra.gov.uk.
Adverse events should also be reported to AbbVie on uk_pvvendor@abbvie.com