This website is for Healthcare Professionals only.
ADVERSE REACTIONS IN THE EPCORE™ NHL-1 TRIAL
CRS-related adverse reactions were predictable, manageable, and resolvable1*
Predictable:
95% of CRS events occurred in the first cycle2
Manageable:
6.6% adverse event–related discontinuation3
- Discontinuation due to CRS or ICANS occurred in 1 patient each (0.6%)3
- 1 patient (0.6%) experienced a fatal adverse reaction (ICANS)3
Resolvable:
CRS resolved in 100%1,3
- Median duration of CRS events was 2 days (range: 0.1-27 days)
Manageable safety profile2
Only 6.6% of patients discontinued subcutaneous TEPKINLY due to adverse reactions.3
Adverse reactions reported in patients in EPCORE™ NHL-13
| System organ class/preferred term or adverse reaction† | All grades | Grade 3-4 |
|---|---|---|
| Infections and infestations | ||
| Viral infectiona | Very common | Common |
| Pneumoniab | Very common | Common |
| Upper respiratory tract infectionc | Common | Common |
| Fungal infectionsd | Common | |
| Sepsise | Common | Common |
| Cellulitis | Common | Common |
| Neoplasm benign, malignant, and unspecified (including cysts and polyps) | ||
| Tumour flare | Common | |
| Blood and lymphatic system disorders | ||
| Neutropeniaf | Very common | Very common |
| Anaemiag | Very common | Very common |
| Thrombocytopeniah | Very common | Common |
| Lymphopeniai | Common | Common |
| Febrile neutropenia | Common | Common |
| Immune system disorders | ||
| Cytokine release syndromej | Very common | Common |
| Metabolism and nutrition disorders | ||
| Decreased appetite | Very common | Uncommon |
| Hypophosphatemia | Common | Common |
| Hypokalemia | Common | Uncommon |
| Hypomagnesemia | Common | |
| Tumour lysis syndromek | Common | Common |
| Nervous system disorders | ||
| Headache | Very common | Uncommon |
| Immune effector cell-associated neurotoxicity syndromej | Common | |
| Cardiac disorders | ||
| Cardiac arrhythmiasl | Very common | Common |
| Respiratory, thoracic, and mediastinal disorders | ||
| Pleural effusion | Common | Common |
| Gastrointestinal disorders | ||
| Abdominal painm | Very common | Common |
| Nausea | Very common | Common |
| Diarrhoea | Very common | |
| Vomiting | Very common | Uncommon |
| Skin and subcutaneous tissue disorders | ||
| Rashn | Common | |
| Pruritus | Common | |
| Musculoskeletal and connective tissue disorders | ||
| Musculoskeletal paino | Very common | Common |
| General disorders and administration site conditions | ||
| Fatiguep | Very common | Common |
| Injection site reactionsq | Very common | |
| Pyrexiar | Very common | Uncommon |
| Oedemas | Very common | Common |
| Investigations | ||
| Alanine aminotransferase increased | Common | Uncommon |
| Aspartate aminotransferase increased | Common | Common |
| Blood creatinine increased | Common | |
| Blood sodium decreasedt | Common | Uncommon |
| Alkaline phosphatase increased | Common | |
Adverse reactions were graded using NCI CTCAE version 5.0.
- TLS has been reported in patients receiving TEPKINLY. Patients at an increased risk for TLS are recommended to receive hydration and prophylactic treatment with a uric acid-lowering agent. Patients should be monitored for signs or symptoms of TLS, especially patients with high tumour burden or rapidly proliferative tumours and patients with reduced renal function1
- The most common adverse reactions (≥20%) were CRS, fatigue, neutropenia, injection site reactions, musculoskeletal pain, abdominal pain, pyrexia, nausea, and diarrhoea1
- Serious adverse reactions occurred in 52% of patients. The most frequent serious adverse reaction (≥10%) was CRS (31%). Seven patients (4.2%) experienced a fatal adverse reaction (pneumonia in 3 [1.8%] patients, viral infection in 3 [1.8%] patients, and ICANS in 1 [0.6%])1
Manageable safety profile: 10.8% patients discontinued subcutaneous TEPKINLY due to adverse reactions4
[SELECT ONLY ONE OF THE FOLLOWING DATA CUTS]
Select adverse events and AEs of special interest reported in patients with R/R DLBCL treated with TEPKINLY in EPCORE NHL-1 (n=139)
| DLBCL, data cut-off: June 30, 2022 | ||
|---|---|---|
| All grades, n (%) | Grade ≥3, n (%) | |
| Neutropenia, 35 (25.2%) | Neutropenia, 24 (17.3%) | |
| Anaemia, 28 (20.1%) | Anaemia, 16 (11.5%) | |
| Thrombocytopenia, 20 (14.4%) | Thrombocytopenia, 8 (5.8%) | |
| Immune effector cell-associated neurotoxicity syndrome, 9 (6.5%) | Immune effector cell-associated neurotoxicity syndrome, 0 (0%) | |
| CRS, 68 (48.9%) | CRS, 5 (3.6%) | |
| Clinical tumour lysis syndrome, 2 (1.4%) | Clinical tumour lysis syndrome, 2 (1.4%) | |
| Fatigue, 33 (23.7%) | Fatigue, 3 (2.2%) | |
| Headache, 18 (12.9%) | Headache, 1 (0.7%) | |
| Nausea, 31 (22.3%) Diarrhoea, 30 (21.6%) Vomiting, 19 (13.7%) | Nausea, 2 (1.4%) Diarrhoea, 0 (0%) Vomiting, 1 (0.7%) | |
| Rash, 9 (6.5%) Pruritus, 9 (6.5%) | Rash, 0 (0%) Pruritus, 0 (0%) | |
| Injection site reactions, 29 (20.9%) Pyrexia, 34 (24.5%) | Injection site reactions, 0 (0%) Pyrexia, 1 (0.7%) | |
Manageable safety profile: 12.2% patients discontinued subcutaneous TEPKINLY due to adverse reactions4
Select adverse events and AEs of special interest reported in patients with R/R DLBCL treated with TEPKINLY in EPCORE NHL-1 (n=139)
| DLBCL, data cut-off: November 18, 2022 | ||
|---|---|---|
| All grades, n (%) | Grade ≥3, n (%) | |
| Neutropenia, 35 (25.2%) | Neutropenia, 24 (17.3%) | |
| Anaemia, 29 (20.9%) | Anaemia, 18 (12.9%) | |
| Thrombocytopenia, 18 (12.9%) | Thrombocytopenia, 7 (5%) | |
| CRS, 69 (49.6%) | CRS, 5 (3.6%) | |
| Immune effector cell-associated neurotoxicity, 9 (6.5%) | Immune effector cell-associated neurotoxicity, 1 (0.7%) | |
| Tumour lysis syndrome, 2 (1.4) | Tumour lysis syndrome, 2 (1.4) | |
| Headache, 18 (12.9) | Headache, 1 (0.7) | |
| Fatigue, 33 (23.7%) | Fatigue, 3 (2.2%) | |
| Nausea, 32 (23.0%) | Nausea, 2 (1.4%) | |
| Abdominal pain, 30 (21.6%) | Abdominal pain, 30 (0%) | |
| Diarrhoea, 30 (21.6%) | Diarrhoea, 0 (0) | |
| Vomiting, 19 (13.7%) | Vomiting, 1 (0.7%) | |
| Rash, 9 (6.5%) | Rash, 0 (0%) | |
| Pruritus, 9 (6.5%) | Pruritus, 0 (0%) | |
| Injection site reactions, 26 (18.7%) | Injection site reactions, 0 (0%) | |
| Pyrexia, 30 (21.6%) | Pyrexia, 0 (0%) | |
Dose delays due to adverse reactions occurred in 32% of patients3
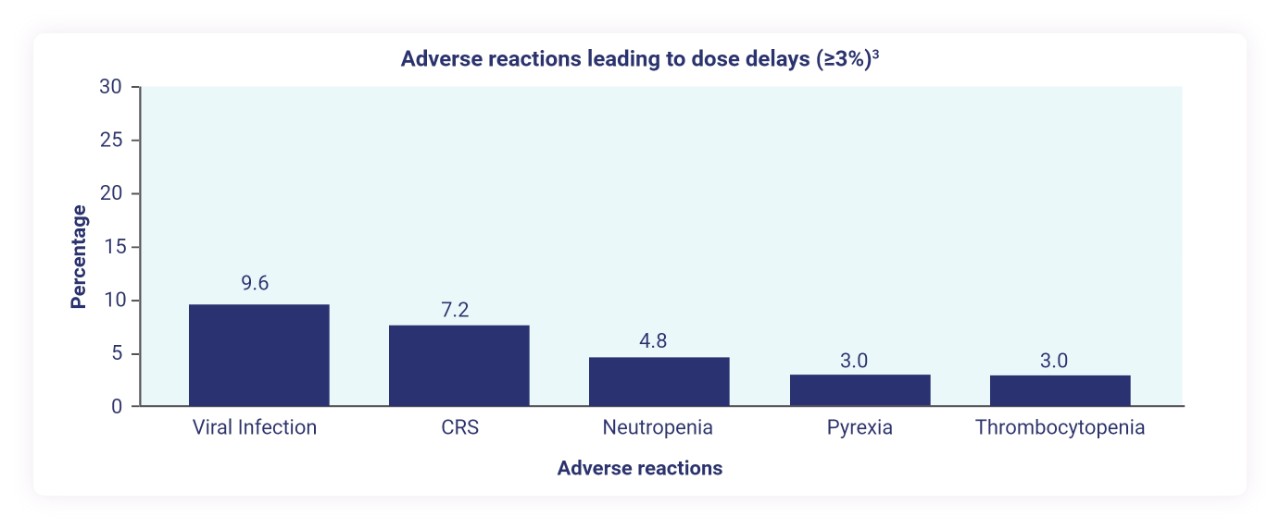
Serious infections occurred in 25% of patients treated with TEPKINLY3‡
- Serious infections, including fatal infections and viral reactivation, have been observed
- Administration of TEPKINLY should be avoided in patients with clinically significant active systemic infections
- The most frequent types of serious infections were COVID-19, COVID-19 pneumonia,* sepsis, upper respiratory infection, bacteraemia, and septic shock
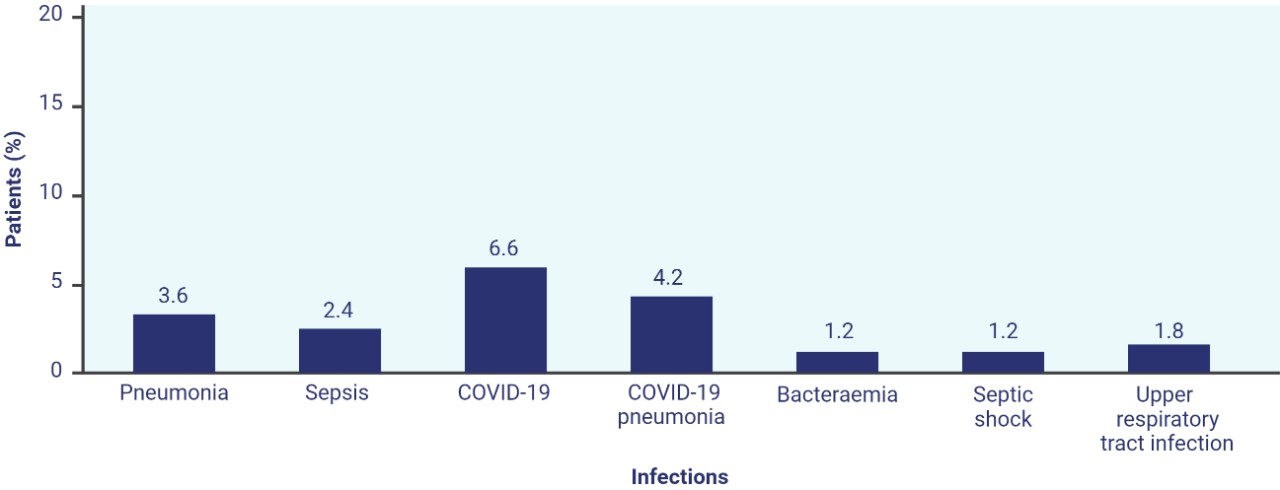
Fatal serious infections occurred in 7 patients (4.2%)
*Actual EPCORE™ NHL-1 study start date: June 26, 2018. Estimated Primary Completion Date: January 2025.5
[INCLUDE SAFETY INFORMATION PER LOCAL REGULATIONS]
I would like to request a meeting with an AbbVie representative.
Learn more about CRS and ICANS events.
C1D1=cycle 1, day 1; C1D8=cycle 1, day 8; C1D15=cycle 1, day 15; C1D22=cycle 1, day 22; C2D1+=cycle 2, days 1+; CRS=cytokine release syndrome; ICANS=immune effector cell-associated neurotoxicity syndrome; NHL=non-Hodgkin lymphoma; TLS=tumour lysis syndrome.
TEPKINLY as monotherapy is indicated for the treatment of adult patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) after two or more lines of systemic therapy.3
References: 1. Thieblemont C, Phillips T, Ghesquieres H, et al. Epcoritamab, a novel, subcutaneous CD3xCD20 bispecific T-cell–engaging antibody, in relapsed or refractory large B-cell lymphoma: dose expansion in a phase I/II trial. J Clin Oncol. Published online December 22, 2022. doi:10.1200/JCO.22.01725 2. Thieblemont C, Phillips T, Ghesquieres H, et al. Epcoritamab, a novel, subcutaneous CD3xCD20 bispecific T-cell–engaging antibody, in relapsed or refractory large B-cell lymphoma: dose expansion in a phase I/II trial. J Clin Oncol. (suppl). Published online December 22, 2022. doi:10.1200/JCO.22.01725 3. TEPKINLY Summary of Product Characteristics. AbbVie Inc. 4. Data on file, AbbVie Inc. 5. First-in-Human (FIH) Trial in Patients with Relapsed, Progressive or Refractory B-Cell Lymphoma (EPCORE™ NHL-1). ClinicalTrials.gov identifier: NCT03625037. Updated May 31, 2023. Accessed June 20, 2023. https://www.clinicaltrialsgov/ct2/show/NCT03625037
ALL-EPCOR-230034

