Current disease management commonly focuses on SYMPTOMS1-3
Current treatments can manage symptoms, but unmet needs remain4
Today’s MF care focuses on:
- Reducing splenomegaly4
- Improving constitutional symptoms4
Management strategies:
- Low- and intermediate-1 risk: Watch and wait; anemia-directed therapy; other symptom-directed therapy based on the clinical situation5
- Intermediate-2 and high-risk: JAK inhibition; ASCT (for select patients)5
- Current guidelines recommend ASCT only for eligible patients who are predicted to have poor survival based on prognostic risk scores (IPSS, DIPSS, and DIPSS Plus)5,6
- In a retrospective study, 3% of MF patients received ASCT7*
- Low- and intermediate-1-risk patients are not routinely offered ASCT5,6
MF response can oftentimes be suboptimal and short-lived8,9
Clinical trial experience
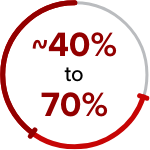
of patients ended JAK inhibition therapy within 3 years of treatment initiation due to loss of response or disease progression8†
Even when patients are on treatment, their disease can continue to progress1
What causes bone marrow fibrosis in MF?10-13
Progressive bone marrow fibrosis can be associated with poor outcomes14,15
Patients may continue to experience disease progression due to the complex mechanisms of this heterogeneous disease8,10
*Retrospective study conducted by a single institution.7
†In a 24-week, placebo-controlled trial, 54.1% of patients with MF did not see an improvement of 50% or more in total symptom score at 24 weeks, even if they experienced reduced symptom burden (N=309).16
‡Based on analyses of 2 large US claims databases, IMS Health® and MarketScan®.9
NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
ASCT=allogeneic stem cell transplantation; DIPSS=Dynamic International Prognostic Scoring System; IMS=Intercontinental Medical Statistics; IPSS=International Prognostic Scoring System; JAK=Janus kinase; JAK/STAT=Janus kinase signal transducer and activator of transcription; MF=myelofibrosis; NCCN=National Comprehensive Cancer Network®.
References: 1. Harrison CN, Schaap N, Mesa RA. Management of myelofibrosis after ruxolitinib failure. Ann Hematol. 2020;99(6):1177-1191. doi:10.1007/s00277-020-04002-9 2. Newberry KJ, Patel K, Masarova L, et al. Clonal evolution and outcomes in myelofibrosis after ruxolitinib discontinuation. Blood. 2017;130(9):1125-1131. doi:10.1182/blood-2017-05-783225 3. Ross DM, Babon JJ, Tvorogov D, Thomas D. Persistence of myelofibrosis treated with ruxolitinib: biology and clinical implications. Haematologica. 2021;106(5):1244-1253. doi:10.3324/haematol.2020.262691 4. Pettit K, Odenike O. Novel therapies for myelofibrosis. Curr Hematol Malig Rep. 2017;12(6):611-624. doi:10.1007/s11899-017-0403-0 5. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Myeloproliferative Neoplasms V.3.2022. © National Comprehensive Cancer Network, Inc. 2022. All rights reserved. To view the most recent and complete version of the guideline, go online to NCCN.org. 6. Devlin R, Gupta V. Myelofibrosis: to transplant or not to transplant? Hematology Am Soc Hematol Educ Program. 2016;2016(1):543-551. doi:10.1182/asheducation-2016.1.543 7. Tefferi A, Lasho TL, Jimma T, et al. One thousand patients with primary myelofibrosis: the Mayo Clinic experience. Mayo Clin Proc. 2012;87(1):25-33. doi:10.1016/j.mayocp.2011.11.001 8. Kuykendall AT, Shah S, Talati C, et al. Between a rux and a hard place: evaluating salvage treatment and outcomes in myelofibrosis after ruxolitinib discontinuation. Ann Hematol. 2018;97(3):435-441. doi:10.1007/s00277-017-3194-4 9. Fonseca E, Silver RT, Kazis LE, Iqbal SU, Rose M, Khan N. Ruxolitinib discontinuation in patients with myelofibrosis: an analysis from clinical practice. Blood. 2013;112(21):2833. doi:10.1182/blood.V122.21.2833.2833 10. Kramann R, Schneider RK. The identification of fibrosis-driving myofibroblast precursors reveals new therapeutic avenues in myelofibrosis. Blood. 2018;131(19):2111-2119. doi:10.1182/blood-2018-02-834820 11. Gleitz HFE, Pritchard JE, Kramann R, Schneider RK. Fibrosis driving myofibroblast precursors in MPN and new therapeutic pathways. HemaSphere. 2019;3(S2):142-145. doi:10.1097/HS9.0000000000000216 12. Gleitz HFE, Kramann R, Schneider RK. Understanding deregulated cellular and molecular dynamics in the haematopoietic stem cell niche to develop novel therapeutics for bone marrow fibrosis. J Pathol. 2018;245(2):138-146. doi:10.1002/path.5078 13. Agarwal A, Morrone K, Bartenstein M, Zhao ZJ, Verma A, Goel S. Bone marrow fibrosis in primary myelofibrosis: pathogenic mechanisms and the role of TGF-β. Stem Cell lnvestig. 2016;3(5):1-10. doi:10.3978/j.issn.2306-9759.2016.02.03 14. Zahr AA, Salama ME, Carreau N, et al. Bone marrow fibrosis in myelofibrosis: pathogenesis, prognosis and targeted strategies. Haematologica. 2016;101(6):660-671. doi:10.3324/haematol.2015.141283 15. Nazha A, Estrov Z, Cortes J, Bueso-Ramos CE, Kantarjian H, Verstovsek S. Prognostic implications and clinical characteristics associated with bone marrow fibrosis in patients with myelofibrosis. Leuk Lymphoma. 2013;54(11):2537-2539. doi:10.3109/10428194.2013.769537 16. Verstovsek S, Mesa RA, Gotlib J, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366(9):799-807. doi:10.1056/NEJMoa1110557
ALL-ONCMF-220048/August 2023
This website is intended for [CountryName] Healthcare Professionals
Copyright ©2023 AbbVie Inc. North Chicago, Illinois, U.S.A.
Unless otherwise specified, all product names appearing in this internet site are trademarks owned by or licensed to AbbVie Inc., its subsidiaries or affiliates. No use of any AbbVie trademark, trade name, or trade dress in this site may be made without the prior written authorization of AbbVie Inc., except to identify the product or services of the company.