SAFETY/TOLERABILITY FOR VENCLYXTO + DECITABINE
DISCONTINUATION RATES WITH VENCLYXTO PLUS DEC
DISCONTINUATIONS, DOSE INTERRUPTIONS, AND DOSE REDUCTIONS DUE TO ARs FOR VEN+DEC1
| • | 26% of patients discontinued treatment |
| • | 65% of patients experienced dose interruption |
| • | 6% of patients had dose reductions |
| • | The most common ARs that led to dose interruptions (≥5%) were febrile neutropaenia, neutropaenia/decreased neutrophil count, pneumonia, decreased platelet count, and decreased white blood cell count |
ADVERSE REACTIONS WITH VENCLYXTO PLUS DEC WERE MANAGEABLE AND WELL-CHARACTERISED1
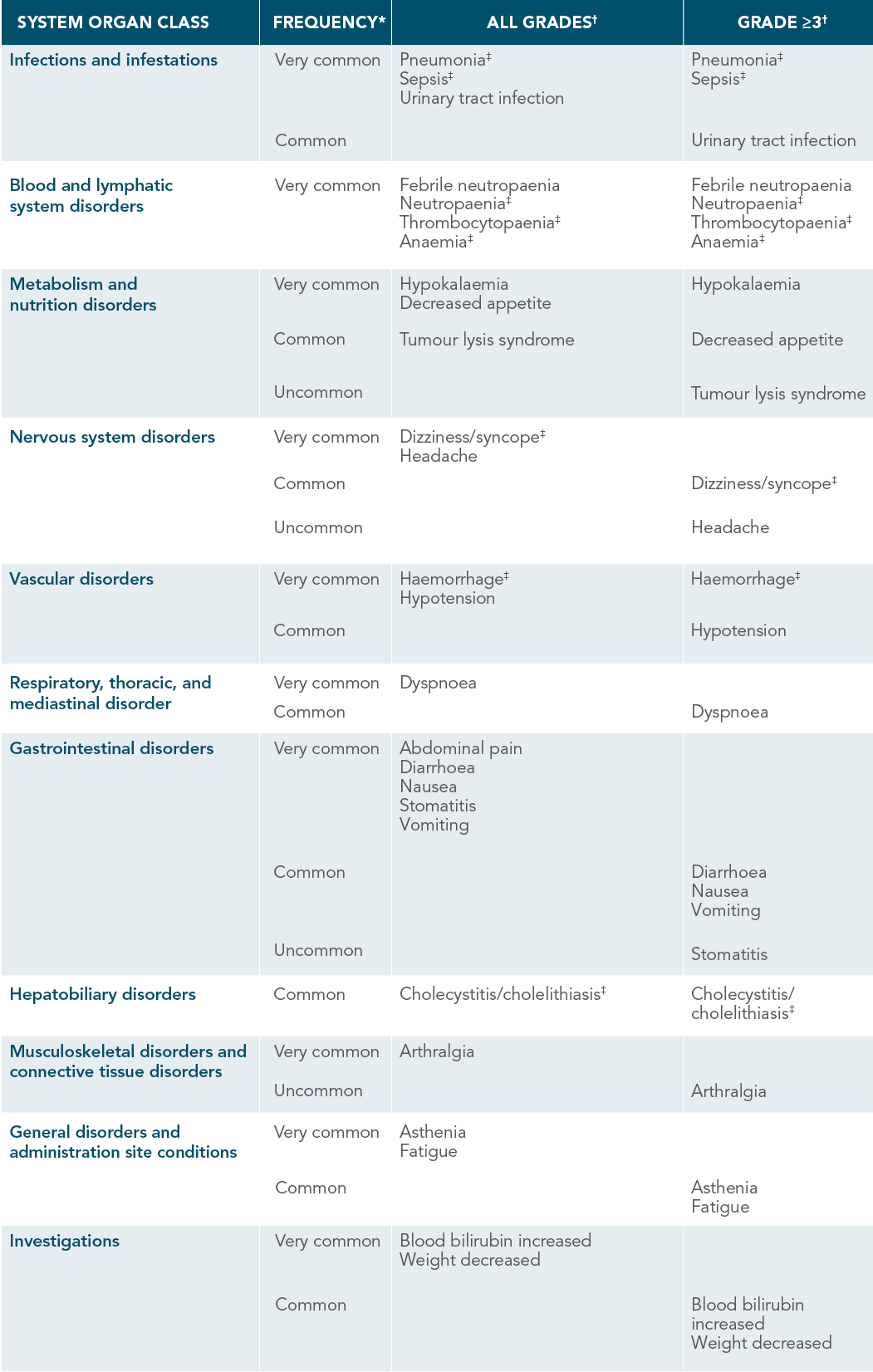
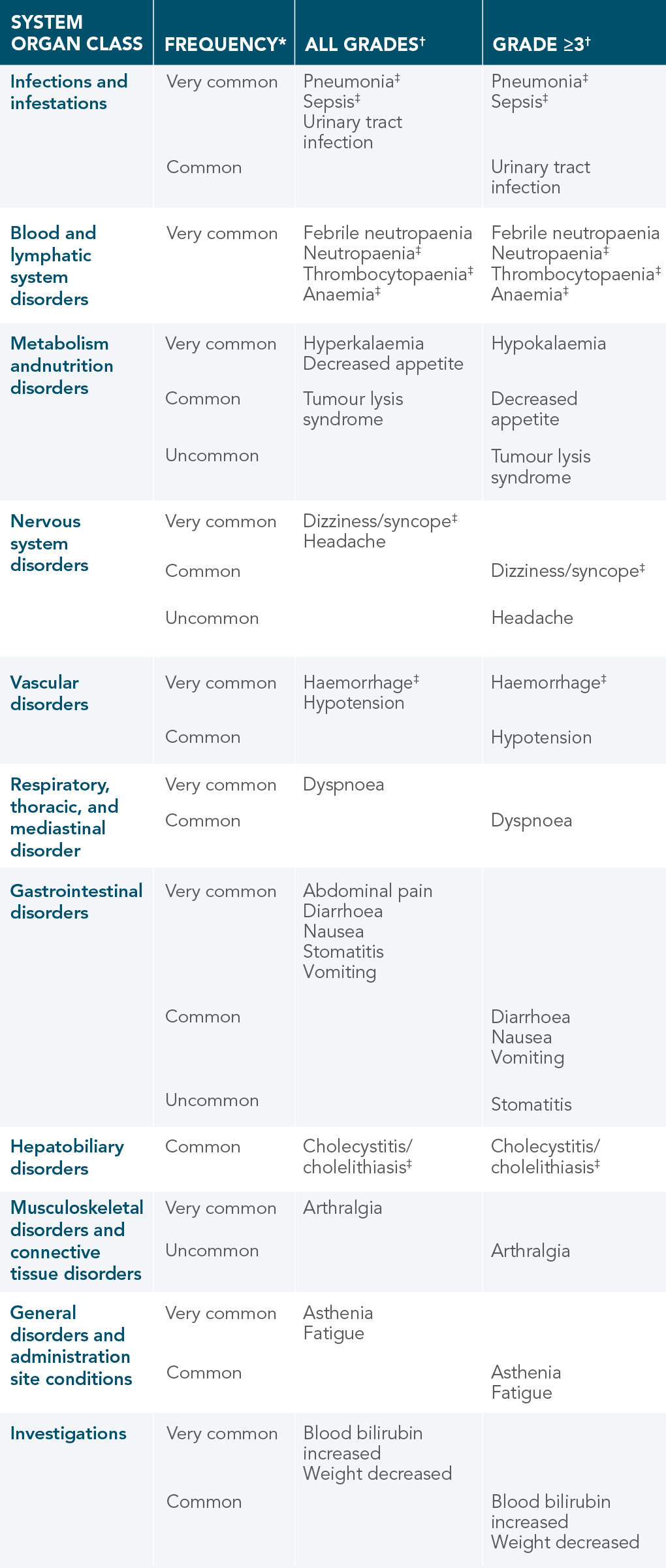
| • | The most commonly occurring ARs (≥20%) of any grade in patients receiving VENCLYXTO plus DEC were thrombocytopaenia, febrile neutropaenia, nausea, haemorrhage, pneumonia, diarrhoea, fatigue, dizziness/syncope, vomiting, neutropaenia, hypotension, hypokalaemia, decreased appetite, headache, abdominal pain, and anaemia |
| • | Most frequently reported serious ARs (≥5%) were febrile neutropaenia, pneumonia, bacteraemia, and sepsis |
| • | No events of laboratory or clinical TLS were reported |
| • | Neutropaenia was reported in 35% (all grades) and 35% (Grade 3 or 4) of patients |
VENCLYXTO ADVERSE DRUG REACTIONS
DEMONSTRATED IN PATIENTS WITH AML1
*Frequencies are defined as very common (≥1/10), common (≥1/100 to <1/10), uncommon (≥1/1000 to <1/100), rare (≥1/10,000 to <1/1000), very rare (<1/10,000), not known (cannot be estimated from available data).1
†Only the highest frequency observed in the trials is reported (based on studies VIALE-A and M14-358).
‡Includes multiple adverse reaction terms.
AML=acute myeloid leukaemia; AR=adverse reaction; BCL-2=B-cell lymphoma 2; CI=confidence interval; CR=complete remission; CRi=complete remission with incomplete haematological recovery; DEC=decitabine; TLS=tumour lysis syndrome; VEN=VENCLYXTO.
[Placeholder for safety balance required by local regulations]
I want to find out
more
about VENCLYXTO
I want to receive more information about VENCLYXTO
Reference: 1. VENCLYXTO Summary of Product Characteristics. Ludwigshafen, Germany: AbbVie Deutschland GmbH & Co. KG. December 2022.
ALL-VNCAML-220066 October 2023