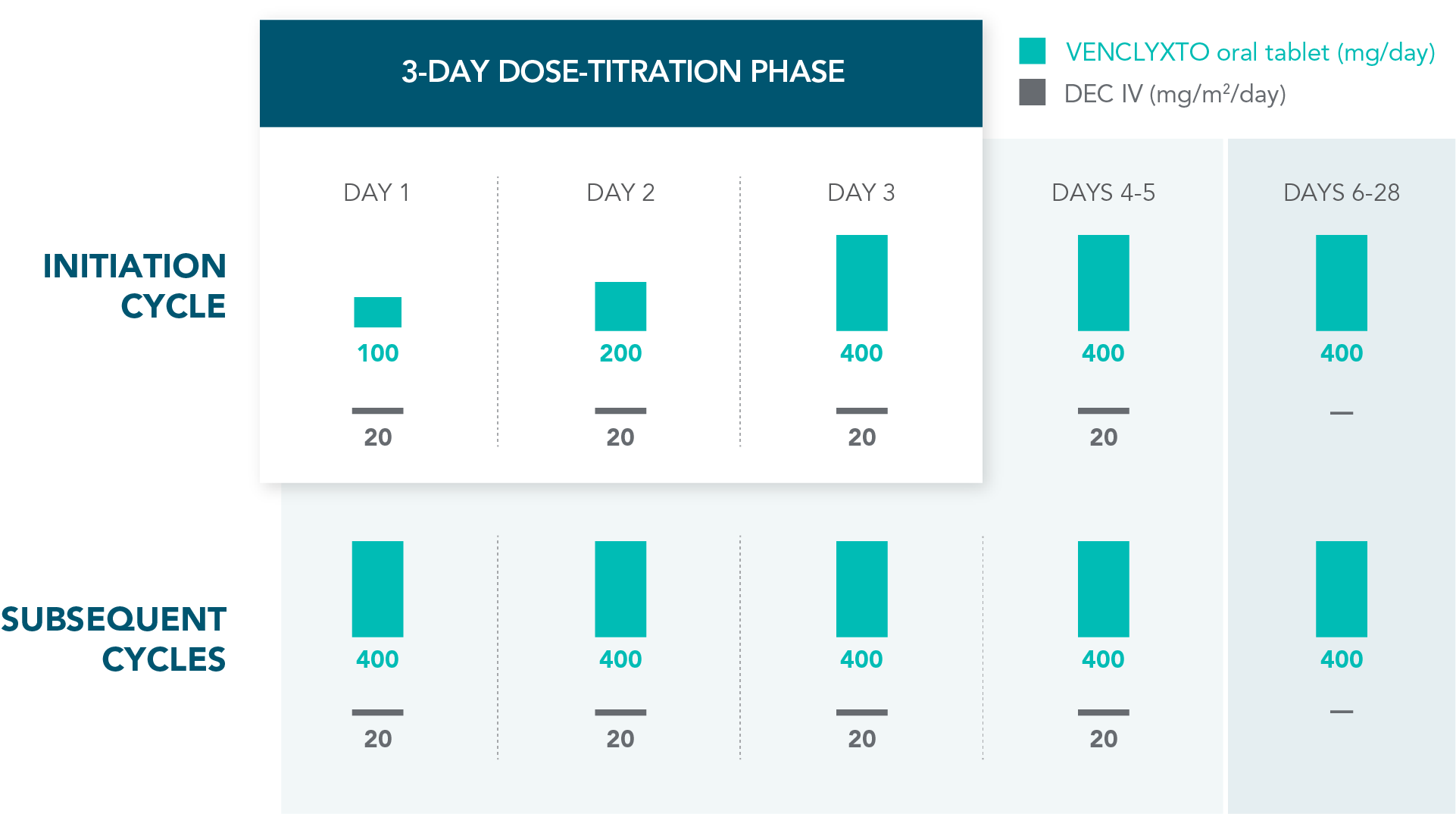
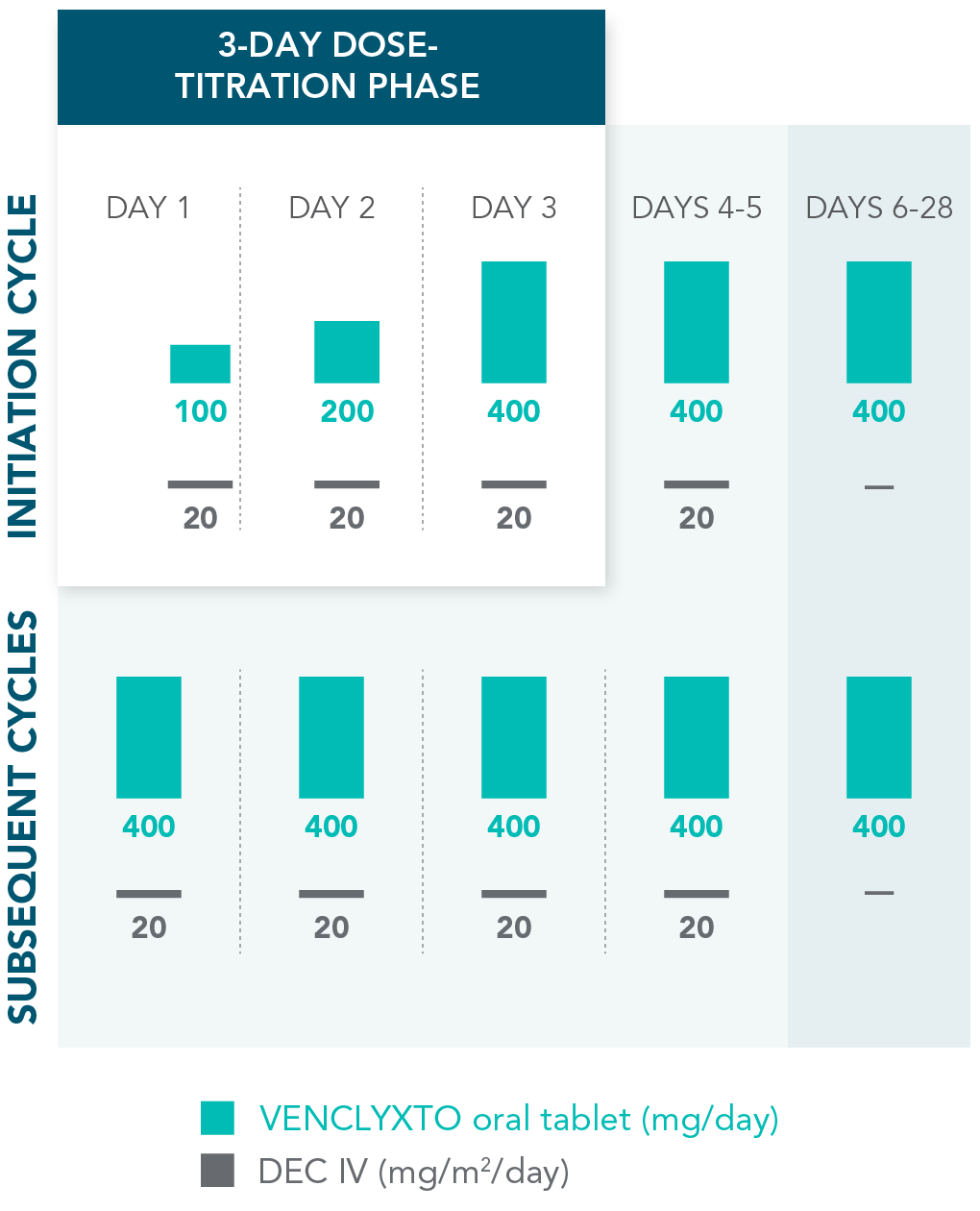
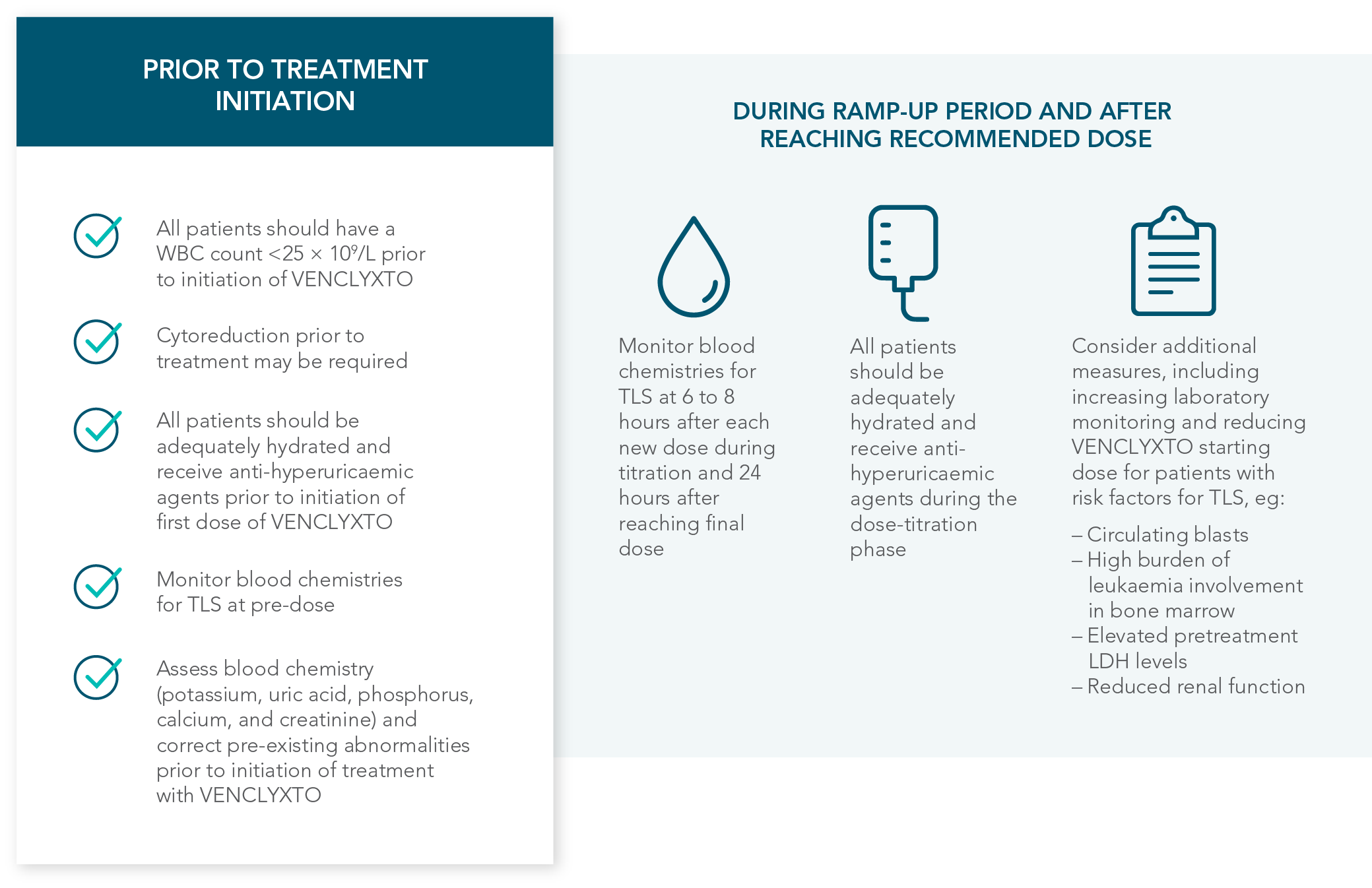
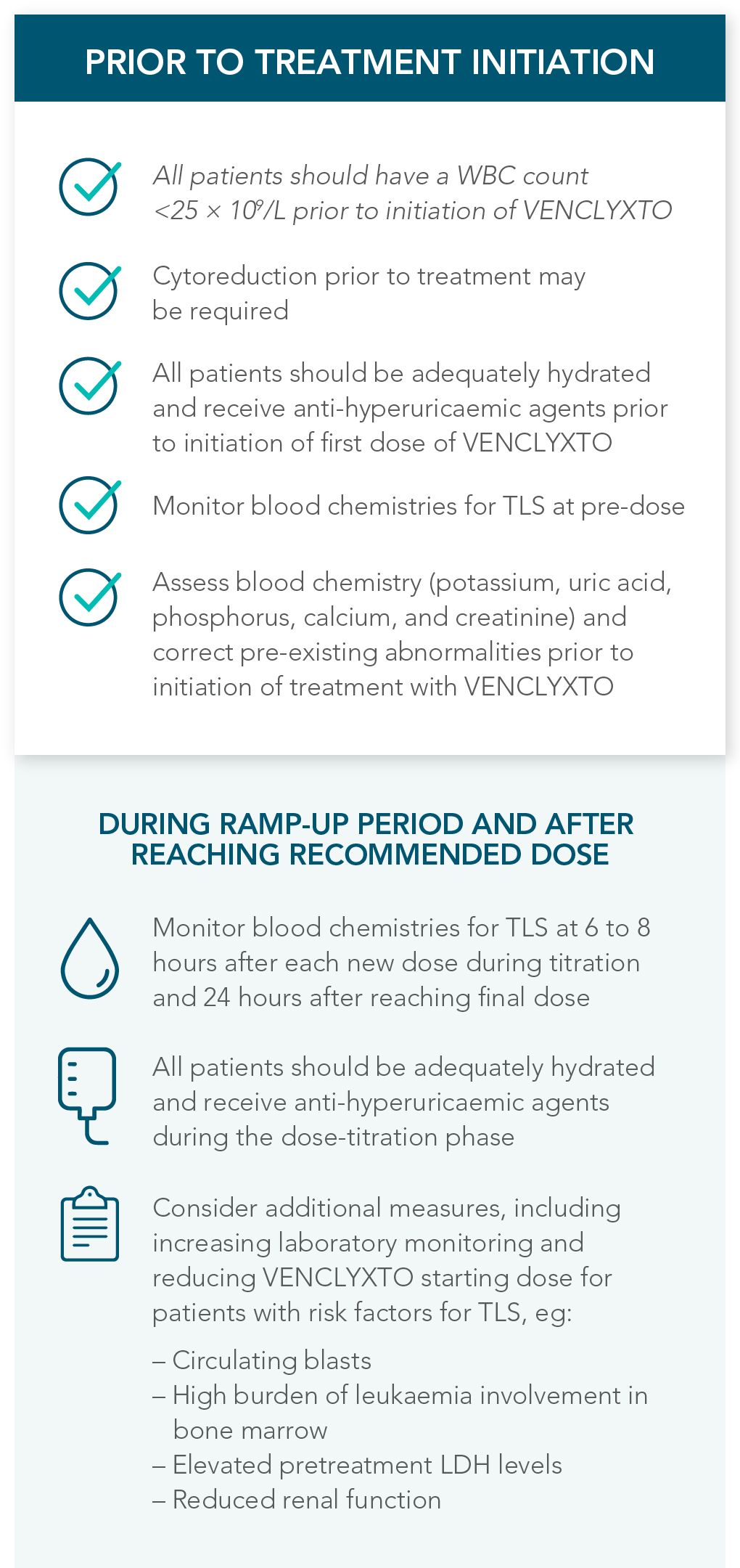
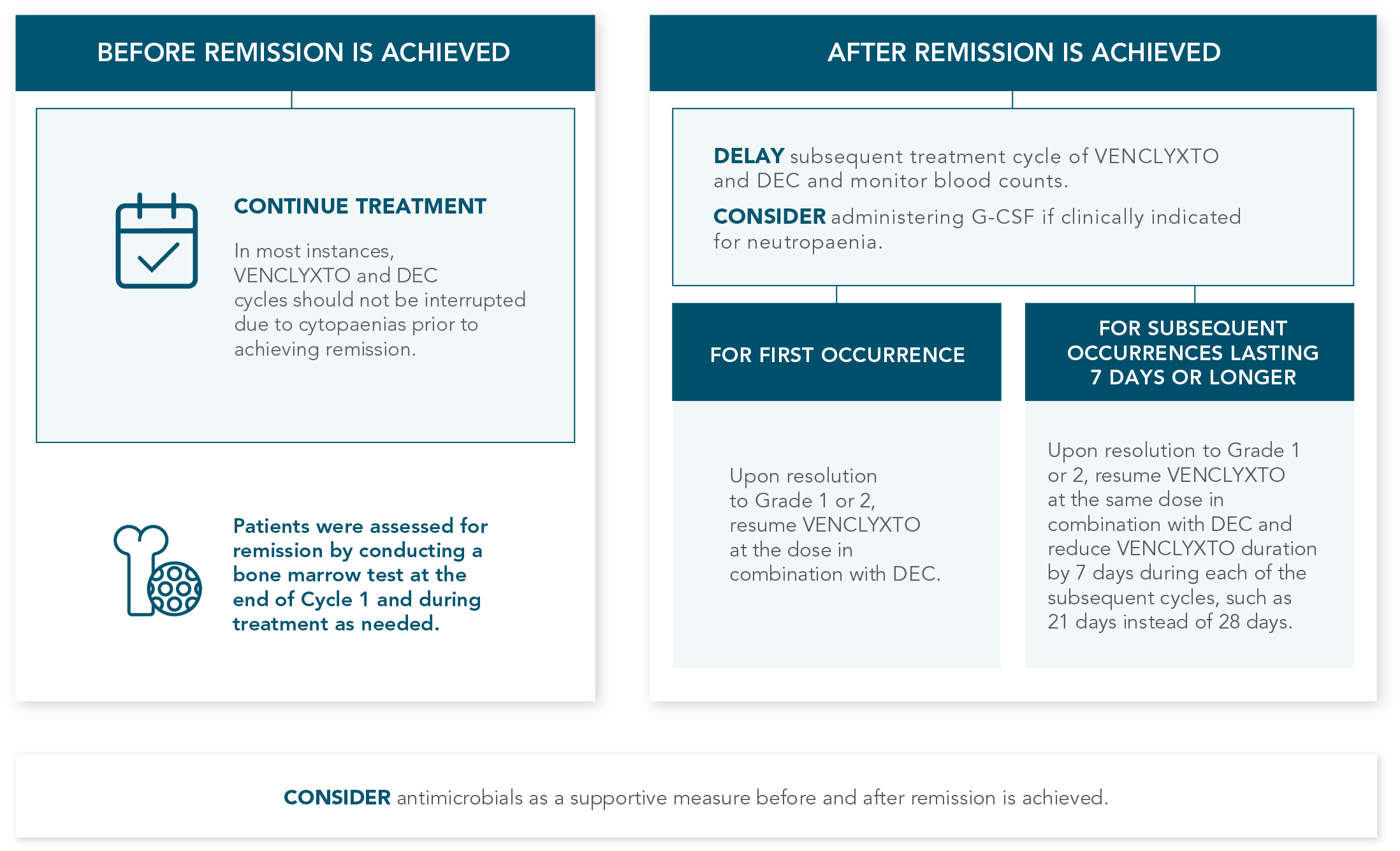
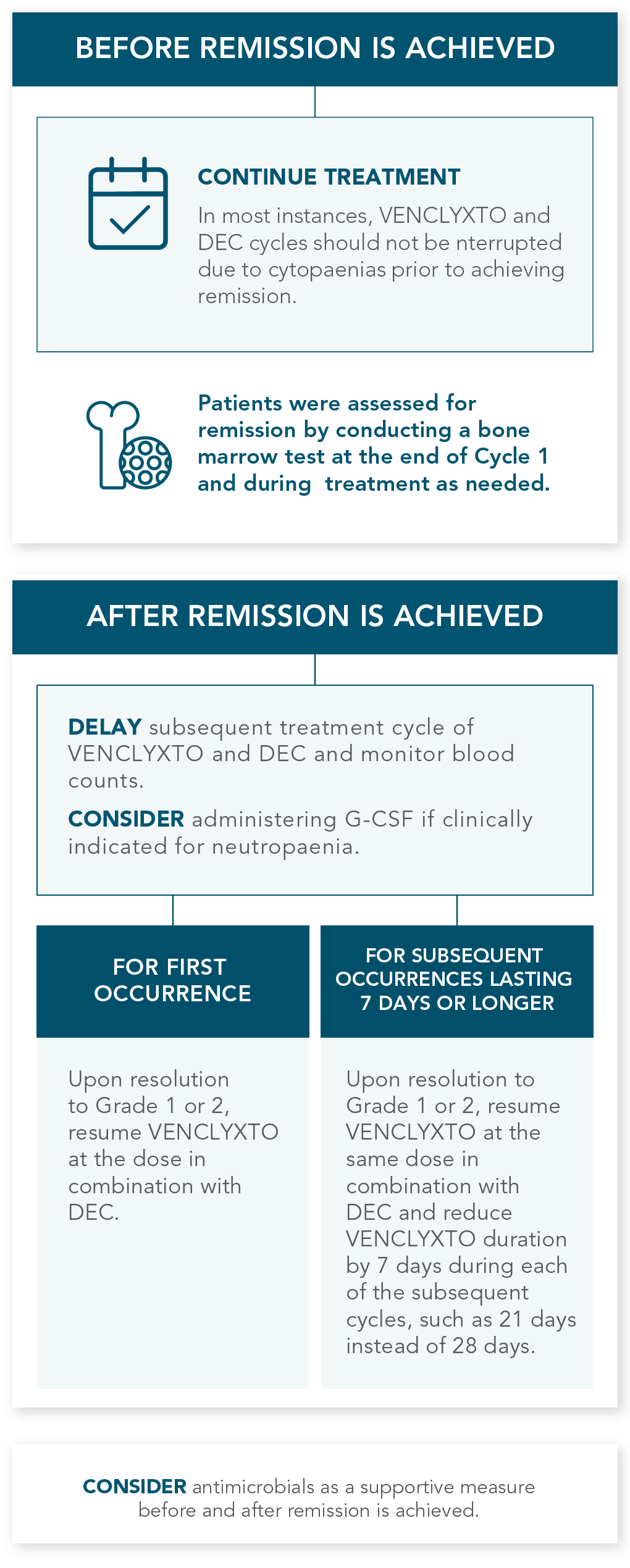
DOSING MANAGEMENT FOR VENCLYXTO + DECITABINE
*Grade 4 neutropaenia (ANC <500/μL) with or without fever or infection; or Grade 4 thrombocytopaenia (platelet count <25,000/μL).1
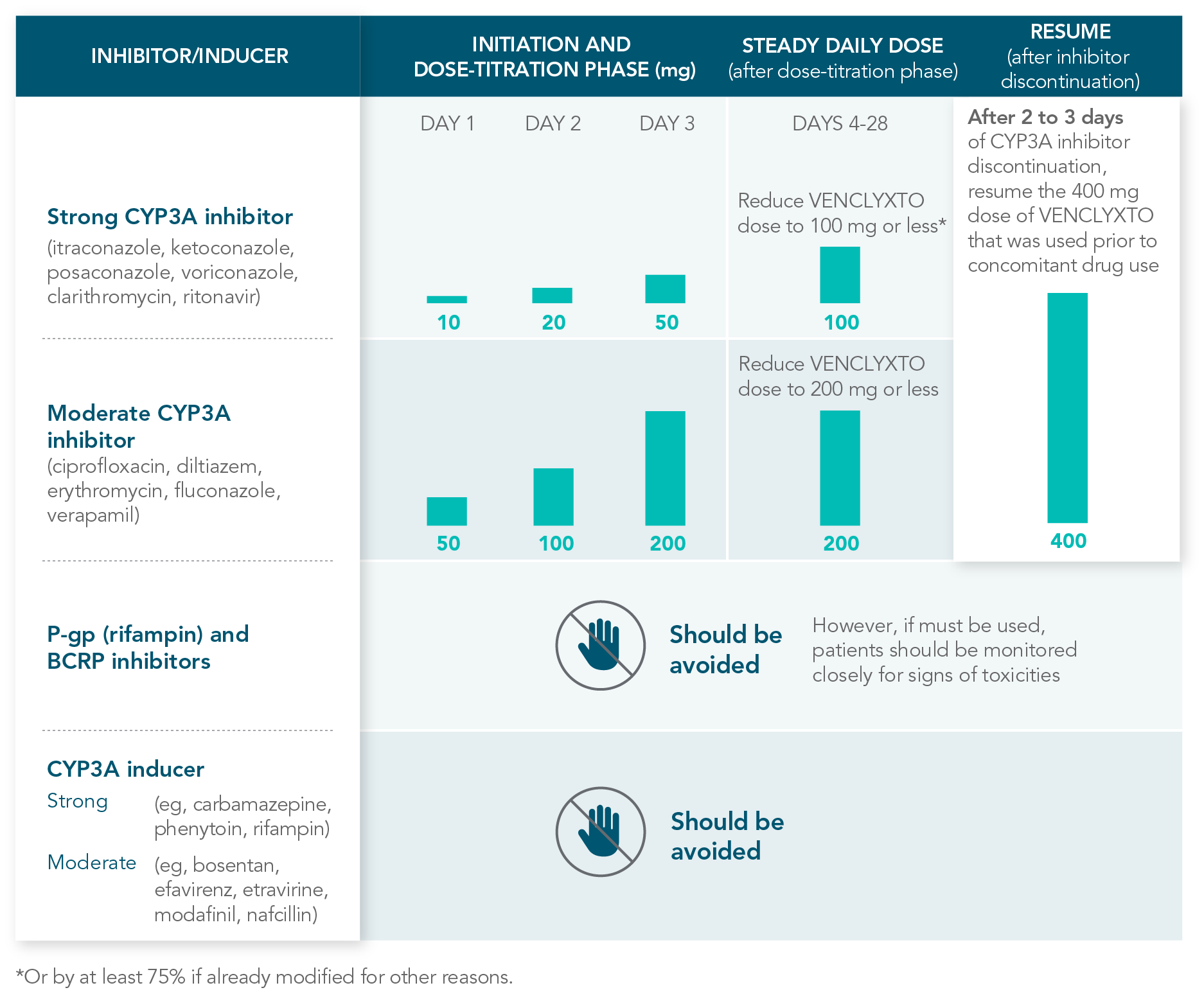
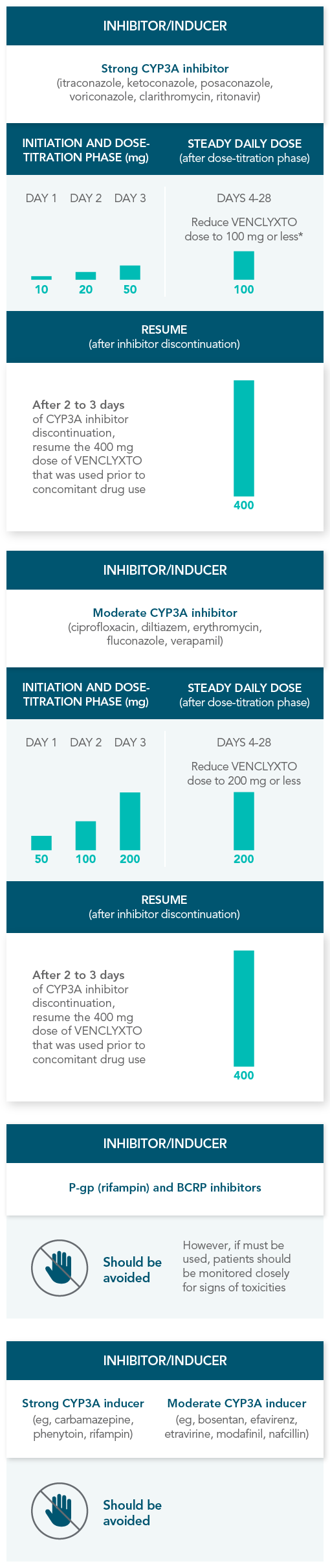
RECOMMENDATIONS FOR THE MANAGEMENT OF CONCOMITANT DRUG INTERACTIONS
If a CYP3A inhibitor must be used, follow the recommended dosing modifications1
DOSE MODIFICATIONS FOR USE WITH CYP3A, P-GP, AND BCRP INHIBITORS AND INDUCERS
Considerations for use with CYP3A inhibitors1
| • | Concomitant use with strong or moderate CYP3A inhibitors increases VENCLYXTO exposure |
| • | Monitor patients closely for signs of toxicities that may require further dose adjustments |
| • | Resume the VENCLYXTO dose used prior to initiating the CYP3A inhibitor 2-3 days after discontinuation of the inhibitor |
AML=acute myeloid leukaemia; ANC=absolute neutrophil count; BCL-2=B-cell lymphoma 2; BCRP=breast cancer resistance protein; CI=confidence interval; CR=complete remission; CRi=complete remission with incomplete haematological recovery; CYP3A=cytochrome P450 3A; DEC=decitabine; G-CSF=granulocyte colony-stimulating factor; IV=intravenous; LDH=lactate dehydrogenase; P-gp=permeability glycoprotein; SC=subcutaneous; TLS=tumour lysis syndrome; WBC=white blood cell.
[Placeholder for safety balance required by local regulations]
I want to find out
more
about VENCLYXTO
I want to receive more information about VENCLYXTO
Reference: 1. VENCLYXTO Summary of Product Characteristics. Ludwigshafen, Germany: AbbVie Deutschland GmbH & Co. KG. December 2022.
ALL-VNCAML-220066 October 2023