*VIALE-A was a randomised (2:1), double-blind, placebo-controlled, Phase 3 study that evaluated the efficacy and safety of VENCLYXTO plus AZA in patients with newly diagnosed AML who were ineligible for intensive chemotherapy. The median overall survival with VENCLYXTO plus AZA was 14.7 months (95% CI: 11.9-18.7) vs 9.6 months for AZA alone (95% CI: 7.4-12.7; HR=0.66; P<0.001).1
DOSING MANAGEMENT FOR VENCLYXTO + AZACITIDINE
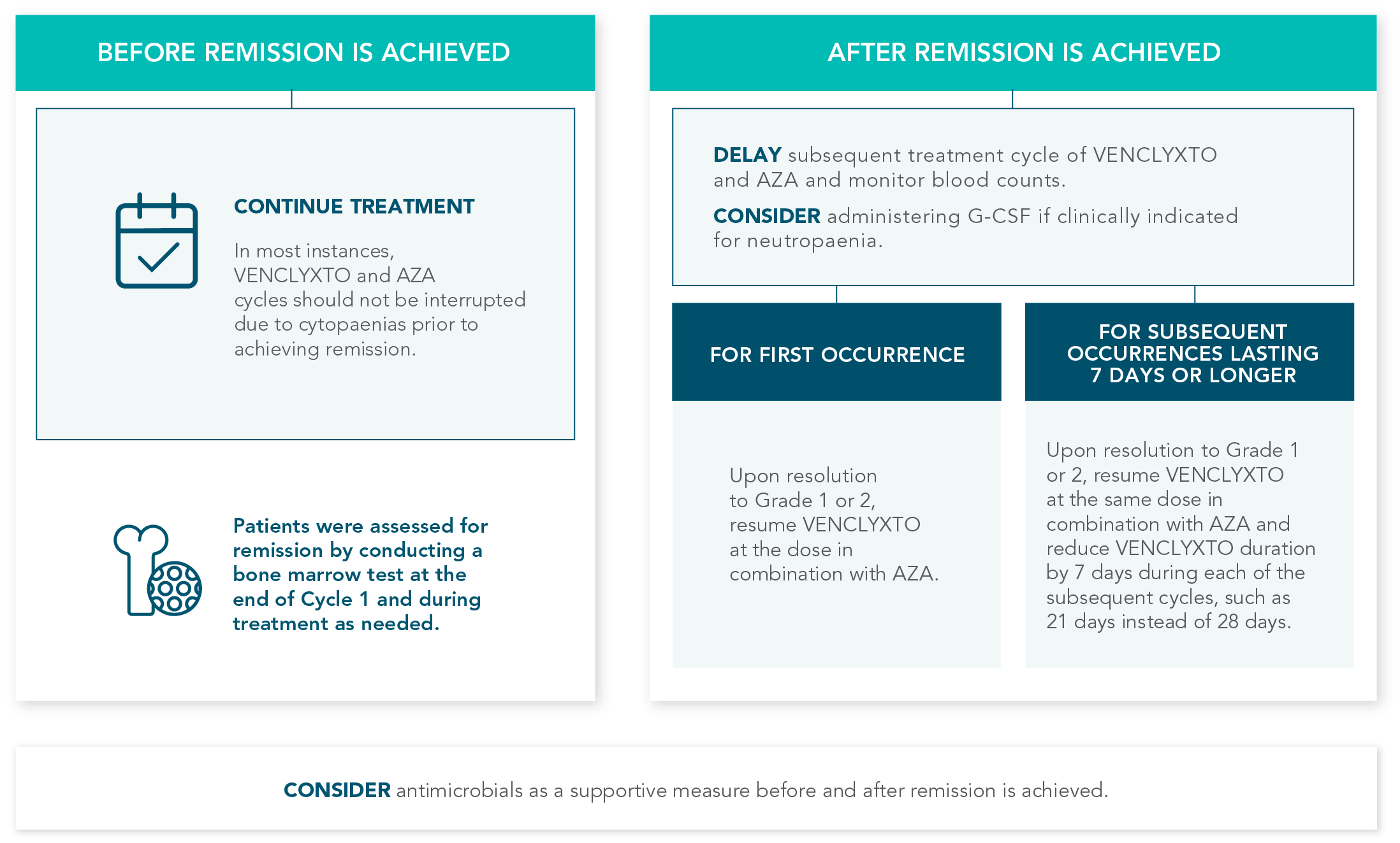
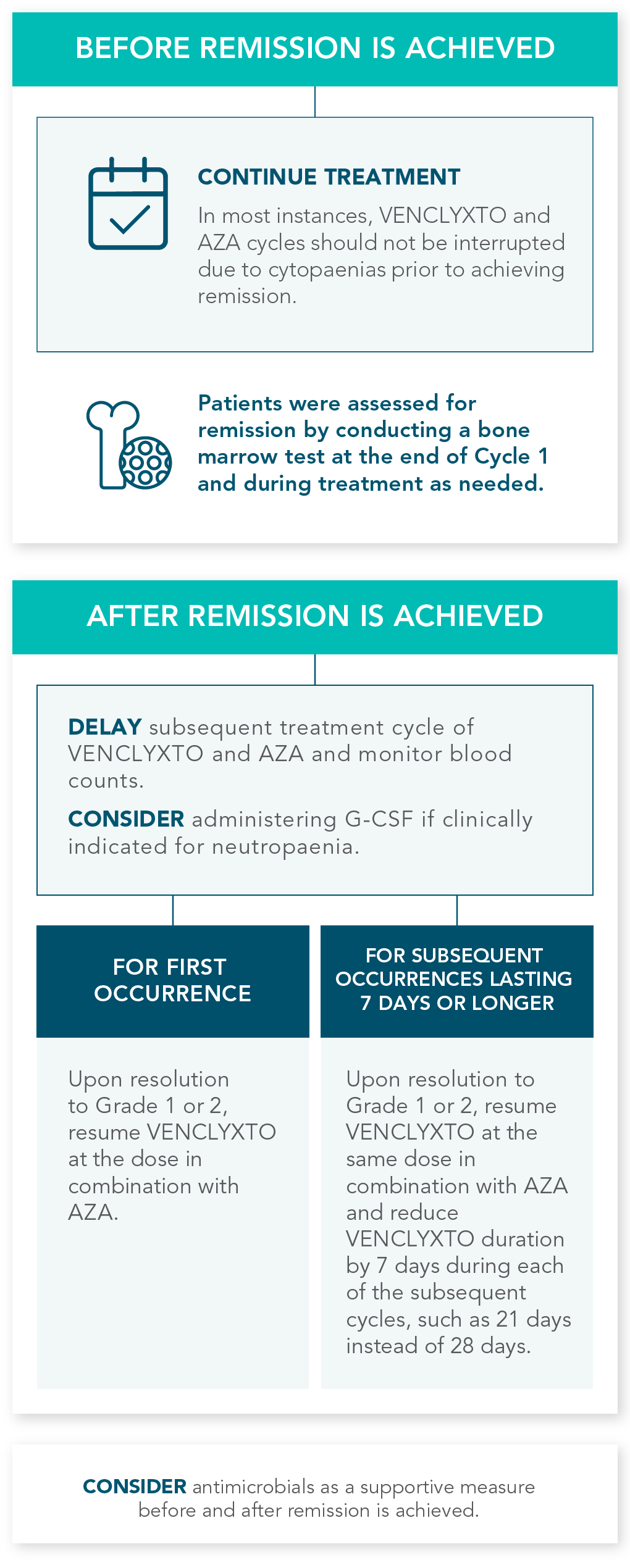
TREATMENT IS MANAGEABLE THROUGHOUT CYCLES OF USE
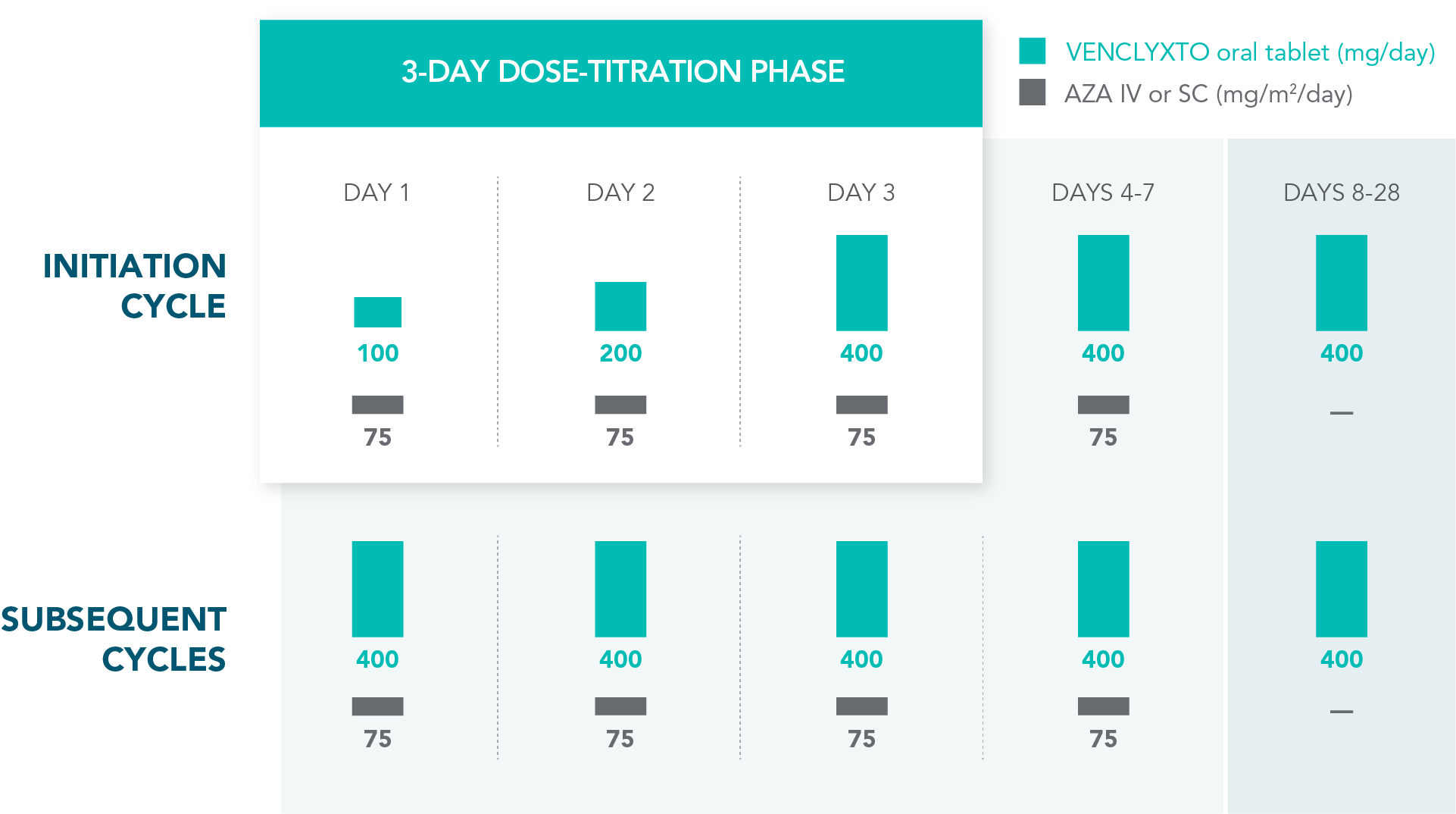
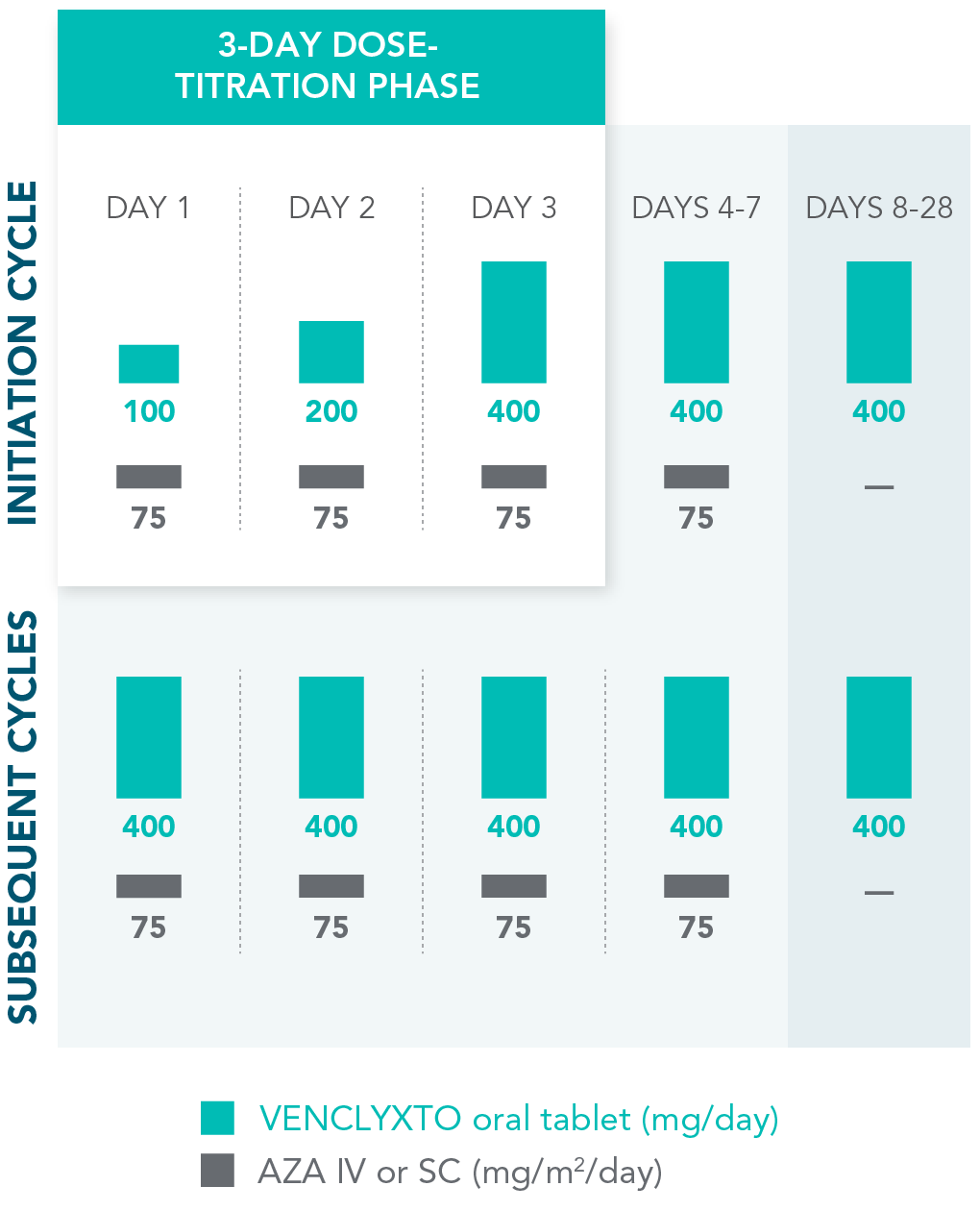
Rapid dose ramp-up safely attains the recommended daily dose1
| • | Dosing schedule was designed to gradually increase exposure to the drug |
DOSING SCHEDULE: VEN+AZA
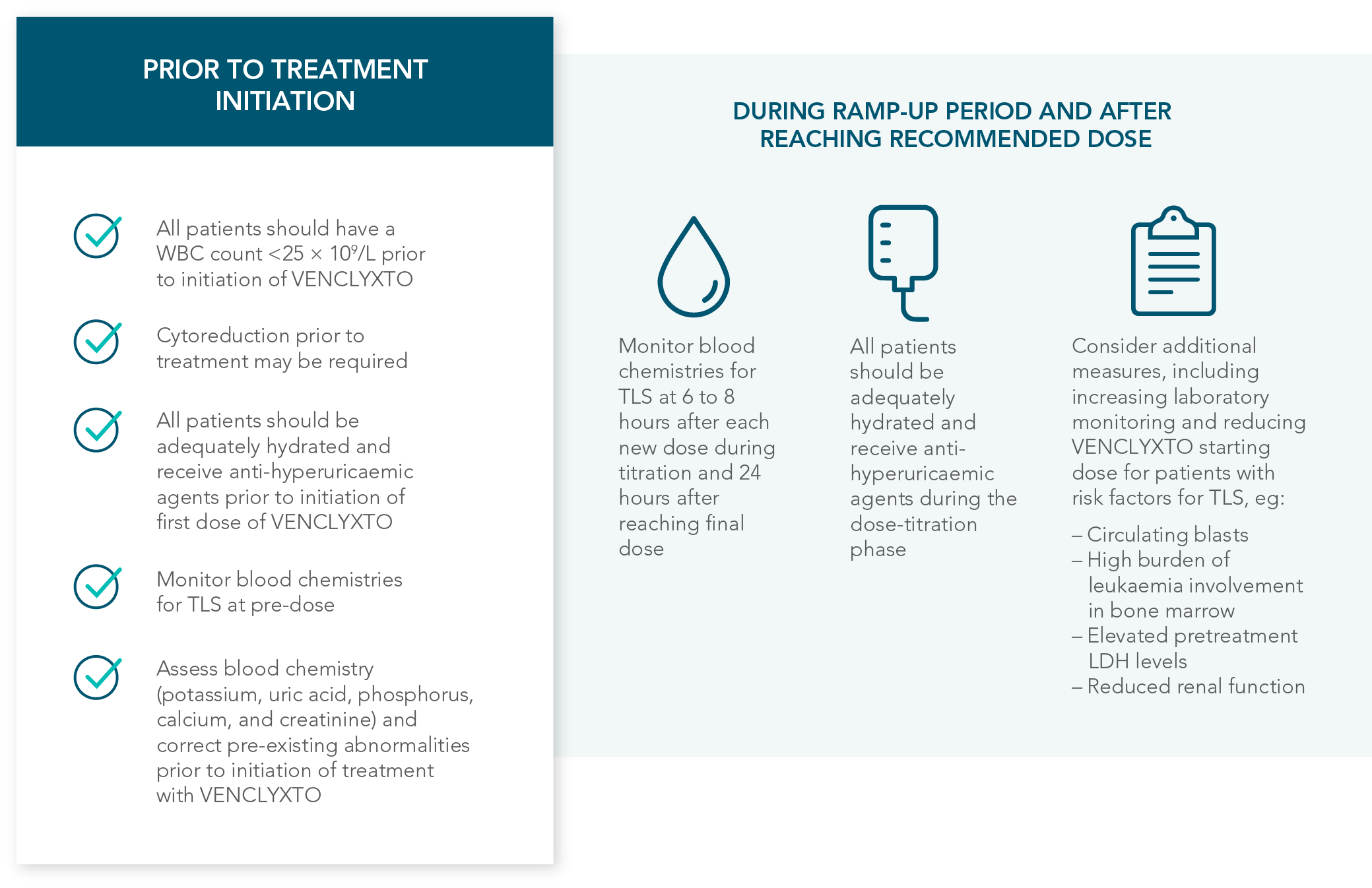
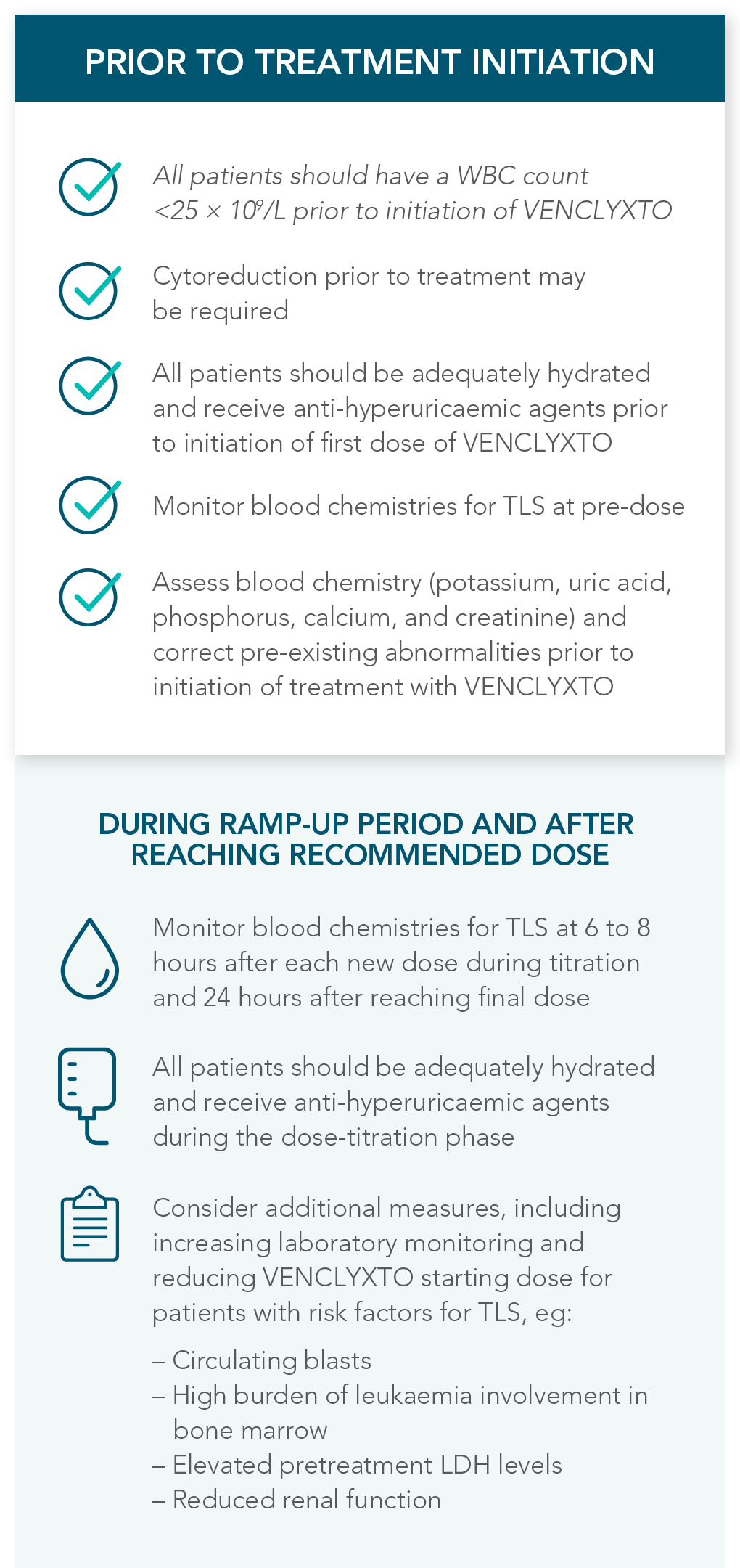
1.1% of patients treated with VENCLYXTO plus AZA experienced TLS, which can be managed by following recommended prophylaxis measures1
| • | 3 patients (1.1%) experienced TLS, 1 of which was clinical TLS |
*Grade 4 neutropaenia (ANC <500/μL) with or without fever or infection; or Grade 4 thrombocytopaenia (platelet count <25,000/μL).1
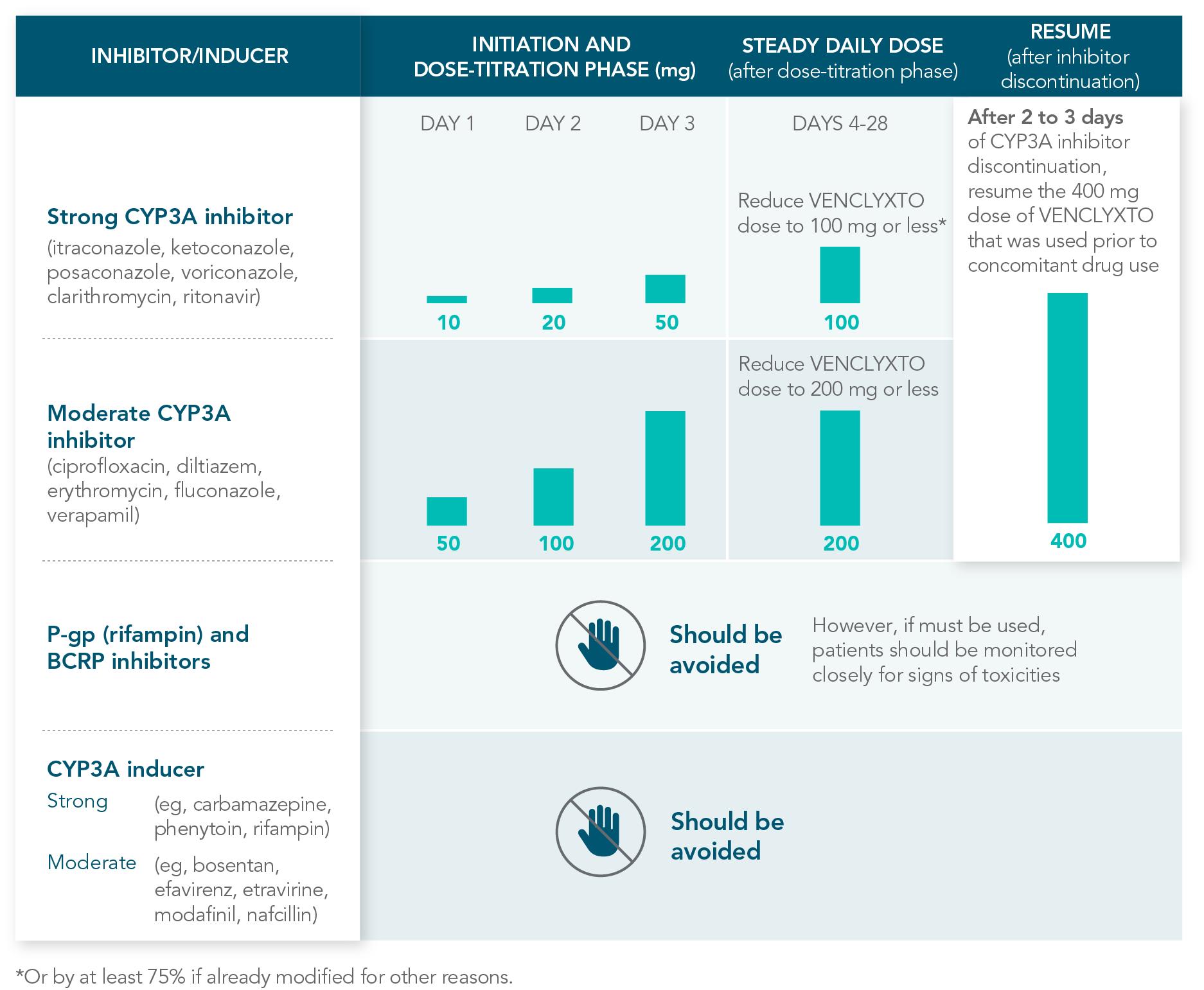
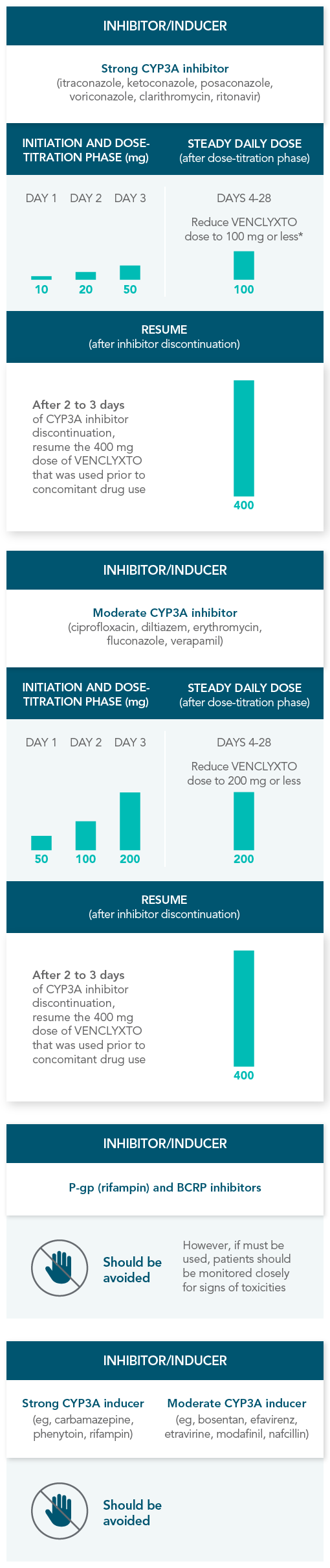
RECOMMENDATIONS FOR THE MANAGEMENT OF CONCOMITANT DRUG INTERACTIONS
If a CYP3A inhibitor must be used, follow the recommended dosing modifications1
DOSE MODIFICATIONS FOR USE WITH CYP3A, P-GP, AND BCRP INHIBITORS AND INDUCERS
Considerations for use with CYP3A inhibitors
| • | Concomitant use with strong or moderate CYP3A inhibitors increases VENCLYXTO exposure |
| • | Monitor patients closely for signs of toxicities that may require further dose adjustments |
| • | Resume the VENCLYXTO dose used prior to initiating the CYP3A inhibitor 2-3 days after discontinuation of the inhibitor |
AML=acute myeloid leukaemia; ANC=absolute neutrophil count; AZA=azacitidine; BCL-2=B-cell lymphoma 2; BCRP=breast cancer resistance protein; CI=confidence interval; CYP3A=cytochrome P450 3A; G-CSF=granulocyte colony-stimulating factor; HR=hazard ratio; IV=intravenous; LDH=lactate dehydrogenase; P-gp=permeability glycoprotein; SC=subcutaneous; TLS=tumour lysis syndrome; VEN=VENCLYXTO; WBC=white blood cell.
[Placeholder for safety balance required by local regulations]
I want to find out
more
about VENCLYXTO
I want to receive more information about VENCLYXTO
Reference: 1. VENCLYXTO Summary of Product Characteristics. Ludwigshafen, Germany: AbbVie Deutschland GmbH & Co. KG. December 2022.
ALL-VNCAML-220066 October 2023