Note to affiliates: This update to the venetoclax CLL AbbVie Pro site includes a homepage headline, updated CLL14 6-year, CLL13 4-year, MURANO 7-year data sets, and other streamlined content updates. CLL 13 4-year update reflects the CLL13 data from the Lancet Oncology publication. The CLL14 6-year and MURANO 7-year data have been updated based on the EHA 2023 abstracts. For countries that cannot use these data sets, please follow local regulations and MRLO guidance, and revert to CLL14 5-year and MURANO 5-year published data from the product label.
Primary analysis in ITT population for VEN+O vs O+Clb1:
INV-assessed PFS†: Reduced risk of progression or death (HR=0.35; 95% CI: 0.23–0.53 [P<0.0001]).
| • | Median follow-up of 28 months |
Additional analyses:
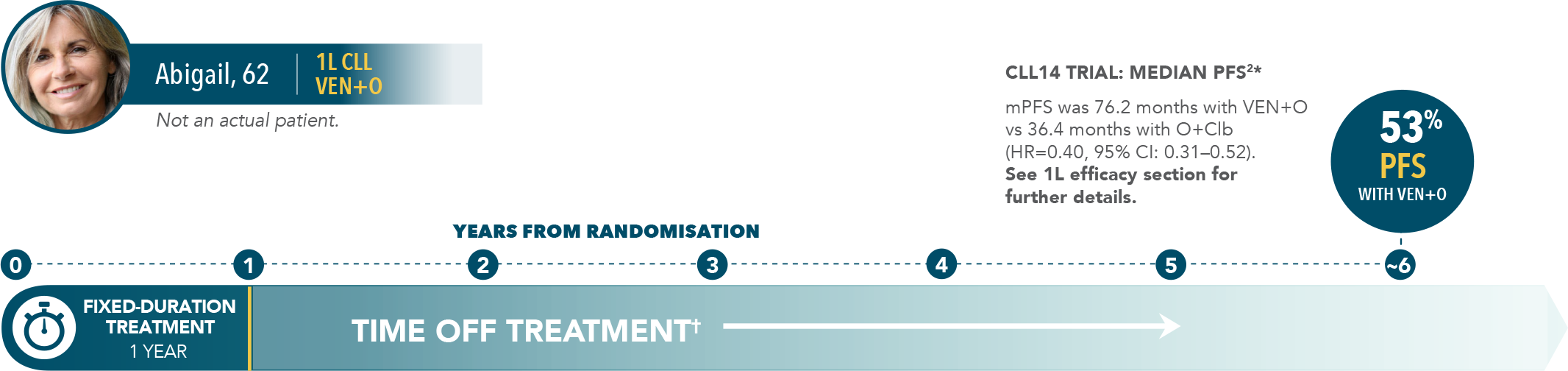
6-year PFS estimate (INV-assessed)2‡: 53% vs 22% (HR=0.40; 95% CI: 0.31–0.52) after 5 years off treatment.
| • | Median PFS of 76.2 months with VEN+O vs 36.4 months with O+Clb |
INV-assessed complete remission (CR/CRi)1: 50% vs 23% (P<0.0001).
| • | ORR: 85% (95% CI: 79.2–89.2) vs 71% (95% CI: 64.8–77.2 [P=0.0007]) |
Primary analysis in ITT population for VEN+R vs BR1:
INV-assessed PFS†: Reduced risk of progression or death (HR=0.17; 95% CI: 0.11–0.25 [P<0.0001]).
| • | Median follow-up of 23.8 months |
Additional analyses:
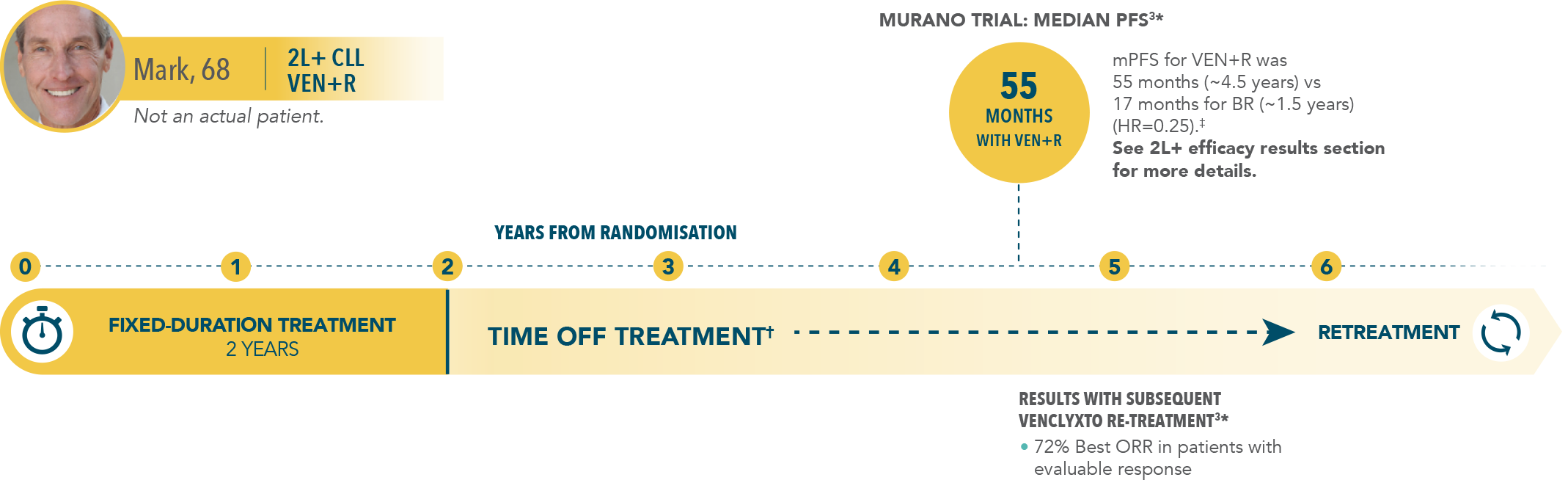
7-year PFS estimate (INV-assessed)3‡: 23% (HR=0.23; 95% CI: 0.18–0.29) vs NE after ~5 years off treatment.
| • | Median PFS of 54.7 months with VEN+R (95% CI: 52.3–59.9) vs 17.0 months with BR (95% CI: 15.5–21.7) |
INV-assessed complete remission (CR/CRi)1‡: 27% vs 8%.
| • | ORR: 93% (95% CI: 88.8–96.4) vs 68% (95% CI: 60.6–74.2) |
*See full dosing information for VEN+O and for VEN+R in the dosing and administration section.
†Primary endpoint.
‡Results are descriptive only.
1L=first line; CLL=chronic lymphocytic leukaemia; VEN+O=VENCLYXTO + obinutuzumab; ITT=intent to treat; O+Clb=obinutuzumab + chlorambucil; INV=investigator; PFS=progression-free survival; HR=hazard ratio; CI=confidence interval; CR=complete remission; CRi=complete remission with incomplete bone marrow recovery; ORR=overall response rate; 2L+=second line + later lines of therapy; VEN+R=VENCLYXTO + rituximab; BR=bendamustine + rituximab; NE=not evaluable.
Note to affiliates:
These are hypothetical treatment journeys reflecting the indicated duration of treatment from the October 2022 EU SmPC. They also include data from the CLL14 6-year and MURANO 7-year follow-up analyses. For those countries that cannot use these data sets, update the content below to highlight the 5-year data for both CLL14 and MURANO.
See study design sections for full clinical trial information.
See the dosing overview section for complete dosing information..
*Results are descriptive
†Based on PPS.
‡Observed in the overall MURANO cohort.
mPFS=medium progression-free survival.
[Placeholder for safety balance required by local regulations]
I want to find out
more
about VENCLYXTO
I want to receive more information
about VENCLYXTO
References: 1. VENCLYXTO Summary of Product Characteristics. Ludwigshafen, Germany: AbbVie Deutschland GmbH & Co. KG.
2. Al-Sawaf O, Robrecht S, Zhang C, et al. Venetoclax-obinutuzumab for previously untreated chronic lymphocytic leukemia: 6-year results
of the randomized CLL14 study. HemaSphere. 2023;7:(S3):1-3. 3. Kater A, Harrup R, Kipps TJ, et al. Final 7-year (yr) follow up and retreatment substudy analysis of MURANO: venetoclax-rituximab (VENR)-treated patients with relapsed/refractory chronic lymphocytic leukemia (R/R CLL). Abstract presented at the European Hematology Association Congress 2023; June 8-11, 2023; Frankfurt, Germany.
ALL-VNCCLL-220060 April 2025