Note to affiliates: This update to the venetoclax CLL AbbVie Pro site includes a homepage headline, updated CLL14 6-year, CLL13 4-year, MURANO 7-year data sets, and other streamlined content updates. CLL 13 4-year update reflects the CLL13 data from the Lancet Oncology publication. The CLL14 6-year and MURANO 7-year data have been updated based on the EHA 2023 abstracts. For countries that cannot use these data sets, please follow local regulations and MRLO guidance, and revert to CLL14 5-year and MURANO 5-year published data from the product label.
Primary analysis in ITT population for VEN+O vs O+Clb1:
INV-assessed PFS†: Reduced risk of progression or death (HR=0.35; 95% CI: 0.23–0.53 [P<0.0001]).
| • | Median follow-up of 28 months |
Additional analyses:
6-year PFS estimate (INV-assessed)2‡: 53% vs 22% (HR=0.40; 95% CI: 0.31–0.52) after 5 years off treatment.
| • | Median PFS of 76.2 months with VEN+O vs 36.4 months with O+Clb |
INV-assessed complete remission (CR/CRi)1: 50% vs 23% (P<0.0001).
| • | ORR: 85% (95% CI: 79.2–89.2) vs 71% (95% CI: 64.8–77.2 [P=0.0007]) |
Primary analysis in ITT population for VEN+R vs BR1:
INV-assessed PFS†: Reduced risk of progression or death (HR=0.17; 95% CI: 0.11–0.25 [P<0.0001]).
| • | Median follow-up of 23.8 months |
Additional analyses:
7-year PFS estimate (INV-assessed)3‡: 23% (HR=0.23; 95% CI: 0.18–0.29) vs NE after ~5 years off treatment.
| • | Median PFS of 54.7 months with VEN+R (95% CI: 52.3–59.9) vs 17.0 months with BR (95% CI: 15.5–21.7) |
INV-assessed complete remission (CR/CRi)1‡: 27% vs 8%.
| • | ORR: 93% (95% CI: 88.8–96.4) vs 68% (95% CI: 60.6–74.2) |
*See full dosing information for VEN+O and for VEN+R in the dosing and administration section.
†Primary endpoint.
‡Results are descriptive only.
1L=first line; CLL=chronic lymphocytic leukaemia; VEN+O=VENCLYXTO + obinutuzumab; ITT=intent to treat; O+Clb=obinutuzumab + chlorambucil; INV=investigator; PFS=progression-free survival; HR=hazard ratio; CI=confidence interval; CR=complete remission; CRi=complete remission with incomplete bone marrow recovery; ORR=overall response rate; 2L+=second line + later lines of therapy; VEN+R=VENCLYXTO + rituximab; BR=bendamustine + rituximab; NE=not evaluable.
PREVENTION OF TUMOUR LYSIS SYNDROME1
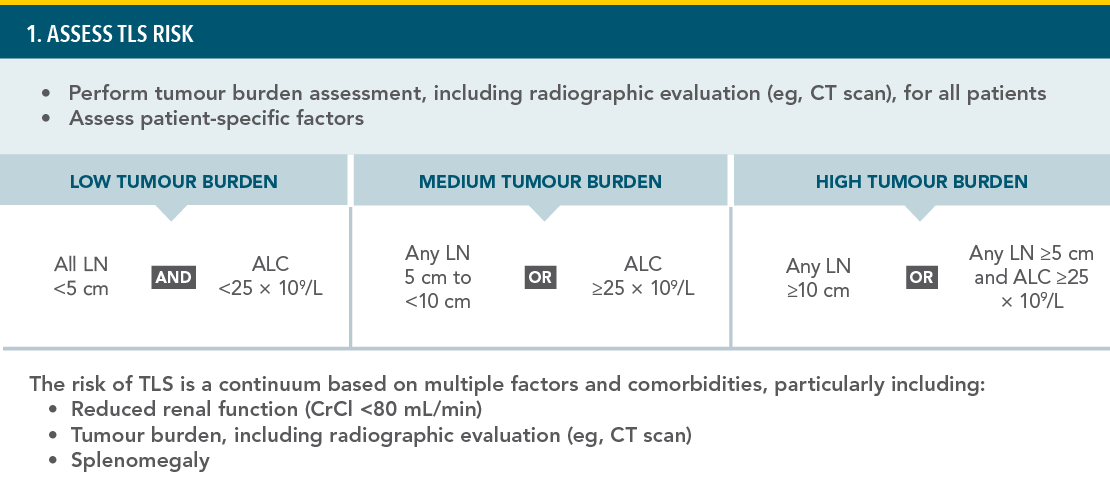
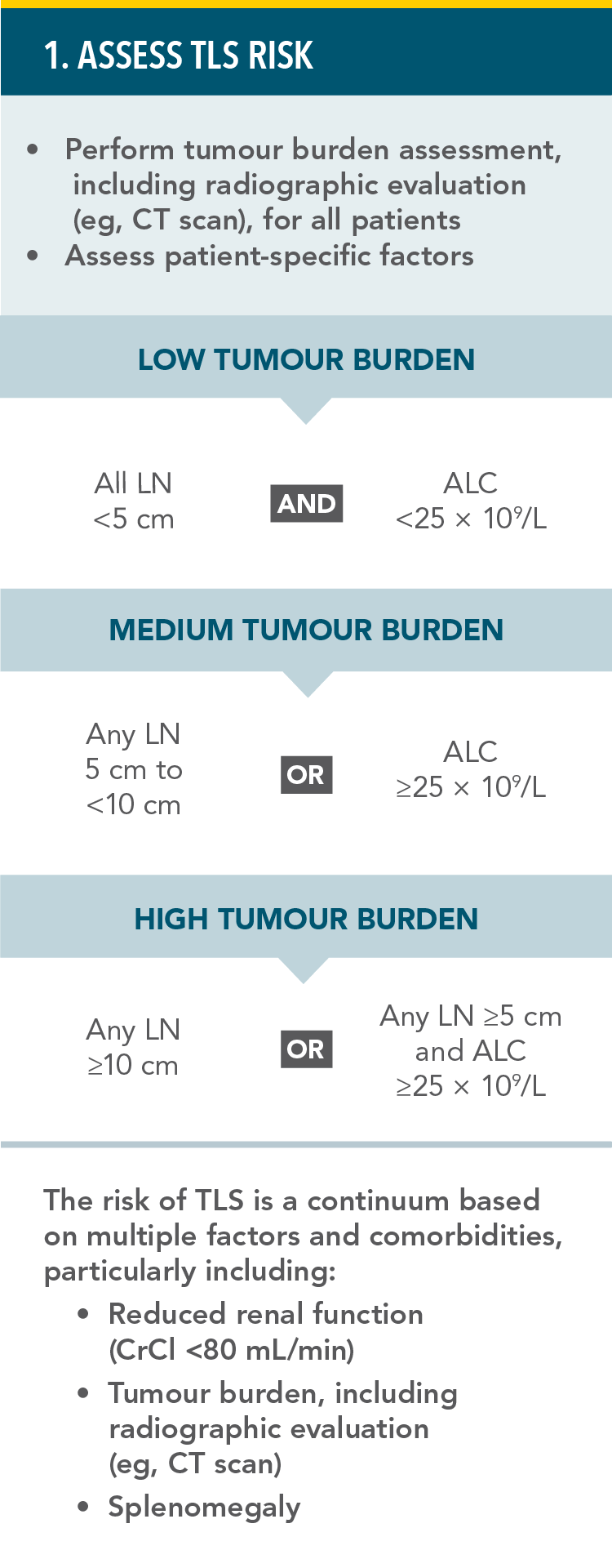
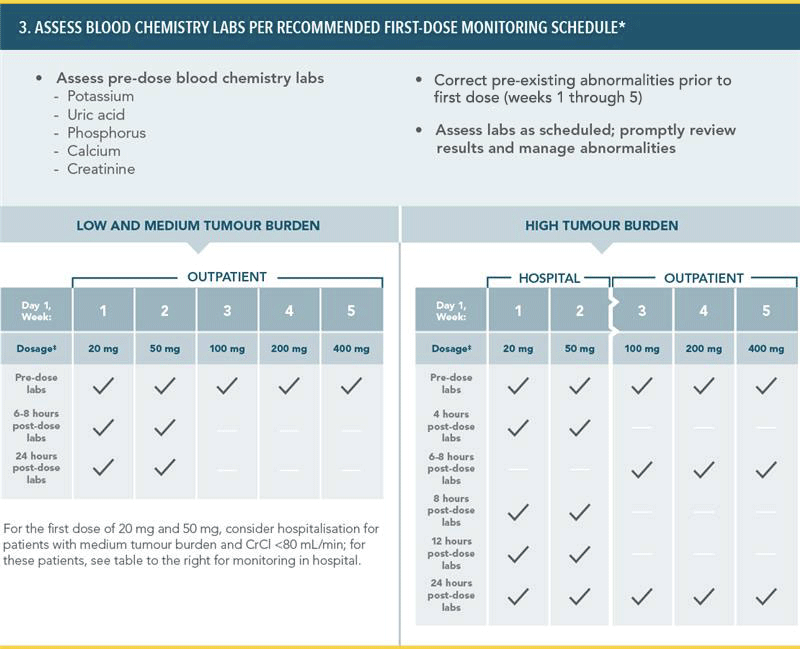
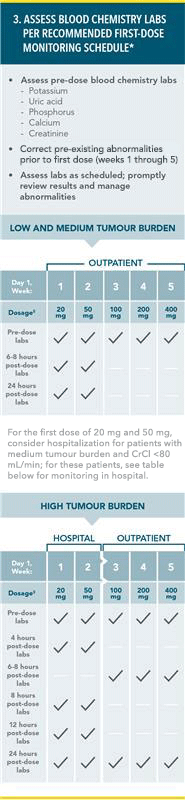
RECOMMENDED TLS PROPHYLAXIS AND MONITORING BASED ON TUMOUR BURDEN IN CLL PATIENTS:
*More intensive measure (IV hydration, frequent monitoring, hospitalisation) should be employed as overall risk increases.
†Administer intravenous hydration for any patient who cannot tolerate oral hydration.
‡For patients at risk of TLS at Week 3, 4, and 5, monitor blood chemistries at 6–8 hours and at 24 hours at each subsequent titration dose.
TLS=tumour lysis syndrome;
CT=computed tomography; LN=lymph node; ALC=absolute lymphocyte count; CrCl=creatinine clearance; IV=intravenous.
[Placeholder for safety balance required by local regulations]
Reference: 1. VENCLYXTO Summary of Product Characteristics. Ludwigshafen, Germany: AbbVie Deutschland GmbH & Co. KG. 2. AI-Sawaf O, Robrecht S, Zhang C, et al. Venetoclax-obinutuzumab for previously untreated chronic lymphocytic leukemia: 6-year results of the randomized CLL14 study. HemaSphere. 2023;7(S3):1-3. 3. Kater A, Harrup R, Kipps TJ, et al. Final 7-year follow up and retreatment substudy analysis of MURANO: venetoclax-rituximab (VENR)-treated patients with relapsed/refractory chronic lymphocytic leukemia (R/R CLL). Abstract presented at the European Hematology Association Congress 2023; June 8-11, 2023; Frankfurt, Germany.
ALL-VNCCLL-220060 April 2025