This website is for Healthcare Professionals only.
CRS & ICANS EVENTS
CRS was primarily low grade, predictable, and manageable1
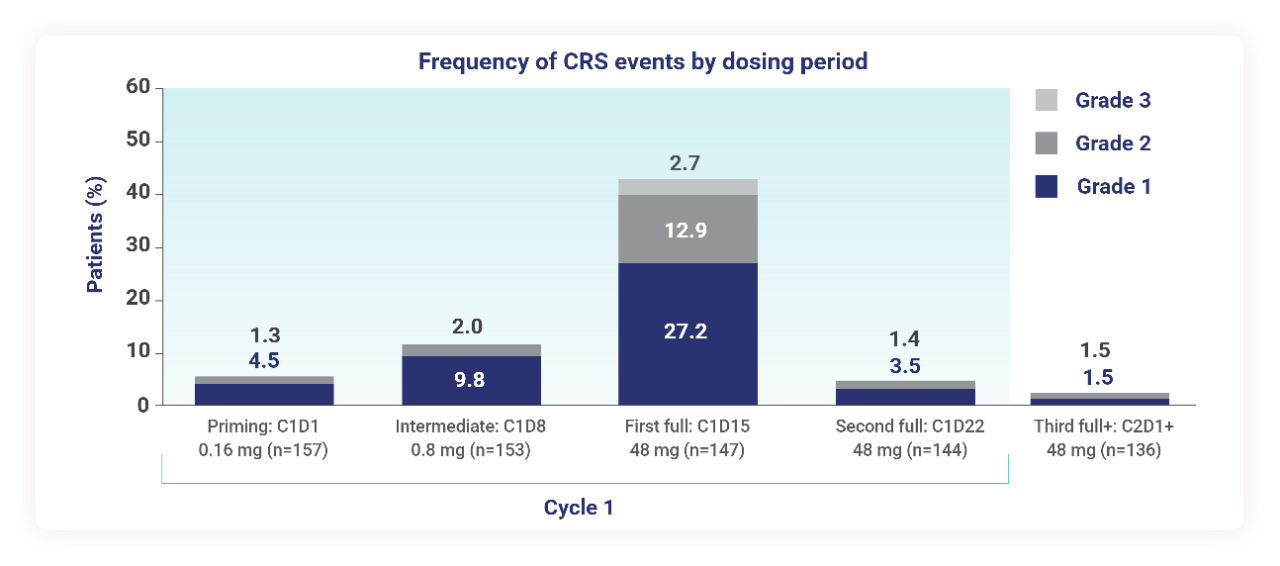
Predictable: 95% of CRS events occurred in the first cycle2
Manageable: 6.6% adverse event–related discontinuation observed. Discontinuation due to CRS or ICANS occurred in 1 patient each (0.6%)3
Resolvable: CRS resolved in 100%3
- Median duration of CRS events was 2 days (range: 0.1-27 days)

- Patients should be monitored for signs and symptoms of CRS and/or ICANS following TEPKINLY administration
- Patients should be hospitalised for 24 hours after administration of the cycle 1, day 15 dose of 48 mg to monitor for signs and symptoms of CRS and/or ICANS
- Patients should be counselled on the signs and symptoms associated with CRS and ICANS and on seeking immediate medical attention should signs or symptoms occur at any time
- Patients should be provided with a patient card and instructed to carry the card at all times. This card describes symptoms of CRS and ICANS which, if experienced, should prompt the patient to seek immediate medical attention
The most common signs and symptoms of CRS include3:
- Pyrexia (99%)
- Hypotension (31%)
- Hypoxia (19%)
ICANS occurred in 6% of patients3
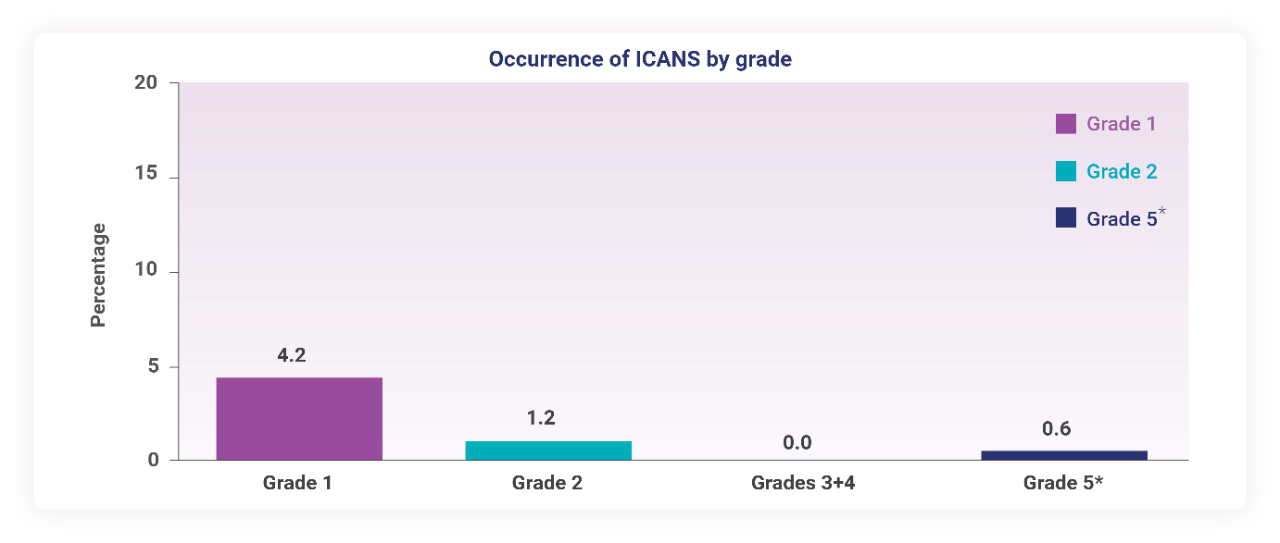
- The median time to first ICANS onset from the start of TEPKINLY treatment was 16.5 days (range: 8-141 days)3
- ICANS resolved in 90% (9/10) of patients with supportive care3
- The median time to resolution of ICANS was 5 days (range: 1-9 days)3
- The onset of ICANS can be concurrent with CRS, following resolution of CRS or in the absence of CRS3
- Treatment-emergent ICANS requiring discontinuation occurred in 1 patient3
- One patient (0.6%) experienced a fatal adverse reaction (ICANS)3
[INCLUDE SAFETY INFORMATION PER LOCAL REGULATIONS]
I would like to request a meeting with an AbbVie representative.
Understand how to manage CRS & ICANS events in your patients.
C1D1=cycle 1, day 1; C1D8=cycle 1, day 8; C1D15=cycle 1, day 15; C1D22=cycle 1, day 22; C2D1+=cycle 2, days 1+; CRS=cytokine release syndrome; ICANS=immune effector cell-associated neurotoxicity syndrome.
TEPKINLY as monotherapy is indicated for the treatment of adult patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) after two or more lines of systemic therapy.3
References: 1. Thieblemont C, Phillips T, Ghesquieres H, et al. Epcoritamab, a novel, subcutaneous CD3xCD20 bispecific T-cell-engaging antibody, in relapsed or refractory large B-cell lymphoma: dose expansion in a phase I/II trial. J Clin Oncol. Published online December 22, 2022. doi:10.1200/JCO.22.01725 2. Thieblemont C, Phillips T, Ghesquieres H, et al. Epcoritamab, a novel, subcutaneous CD3xCD20 bispecific T-cell–engaging antibody, in relapsed or refractory large B-cell lymphoma: dose expansion in a phase I/II trial. J Clin Oncol. (suppl). Published online December 22, 2022. doi:10.1200/jco.22.01725 3. TEPKINLY Summary of Product Characteristics. AbbVie Inc.
ALL-EPCOR-230034

