Note to Affiliate: This document is based on the November 2023 Irish SmPC, the August 2020 J Tube IFU, the March 2019 NJ Tube IFU, the August 2020 PEG IFU, the 2019 CADD Duodopa Patient Information Guide, and the February 2019 CADD Duodopa Pump Operator's Manual.
Because ON TIME is their time
Patient checklist
Duodopa is for the treatment of advanced levodopa-responsive Parkinson’s disease with severe motor fluctuations and hyperkinesia or dyskinesia when available combinations of Parkinson medicinal products have not given satisfactory results1
Clinical indicators of advanced Parkinson’s disease
A consensus was developed with a panel of 17 movement disorder specialists from 10 countries and 15 indicators of suspected advanced Parkinson’s disease were developed2*
Screening tool
Among the 15 indicators, 3 have been proposed as a screening tool – the 5-2-1 criteria:
- Taking at least 5 oral levodopa doses per day
- Having at least 2 hours of ‘OFF’ time per waking day
- Having at least 1 hour of troublesome dyskinesia per waking day
The presence of at least one of these indicators may be a sign of advanced Parkinson’s disease
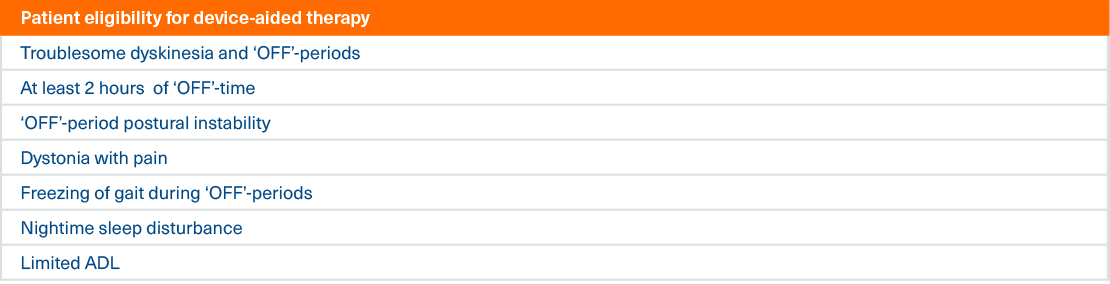
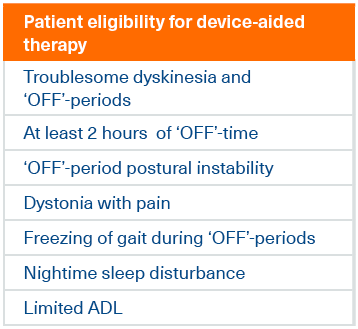
Clinical indicators for device-aided therapy
The same group of experts refined the indicators further into 7 characteristics of people with advanced Parkinson’s disease that would
make them eligible for device-aided therapy such as Duodopa2
These criteria may act as a useful checklist when you are identifying patients that may be eligible for Duodopa therapy
The presence of any one of these indicators (except limited ADL, which would require the presence of another indicator) may indicate device-aided therapy eligibility2
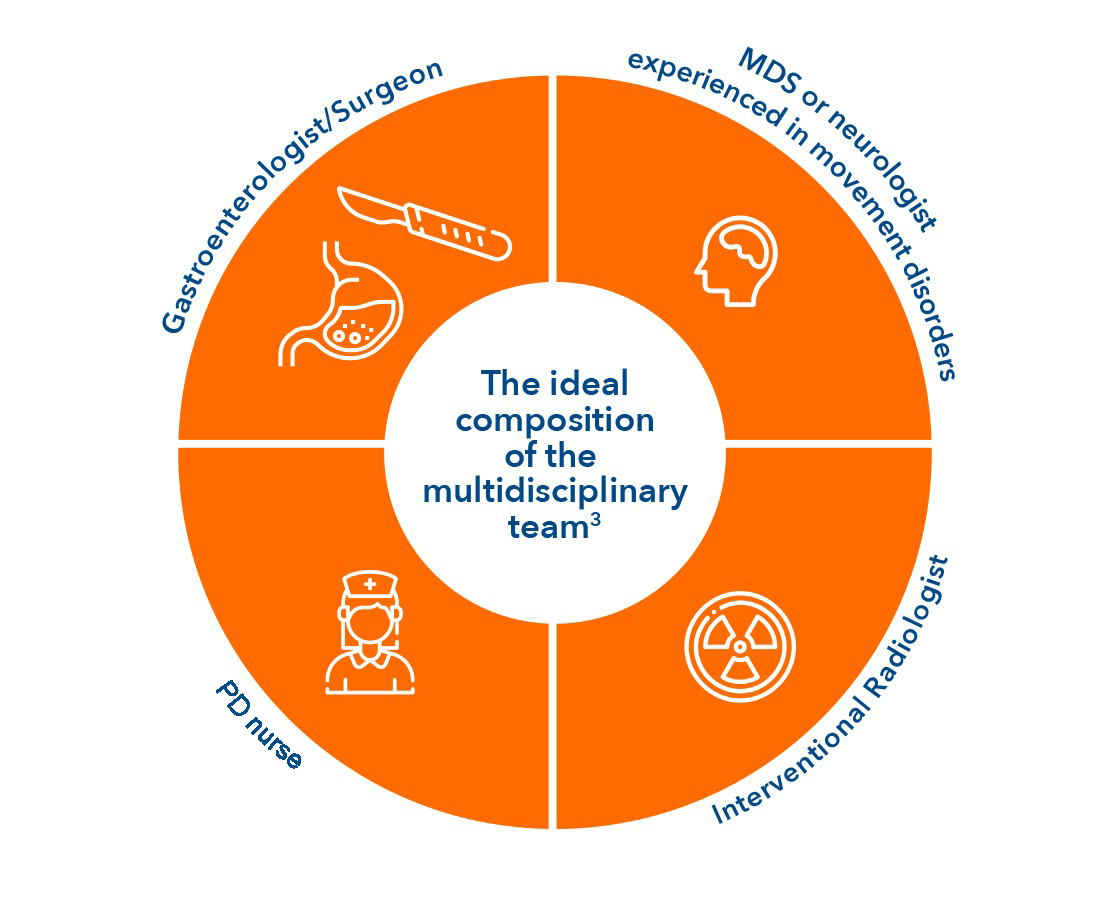
Collaboration and teamwork are important
A collaborative and clearly structured multidisciplinary team (MDT) is key to optimal delivery of Duodopa3
Local practice may also determine the involvement of other healthcare professionals in the MDT4
Effective communication and collaborative decision-making can improve patient health and satisfaction while a failure to do so can adversely affect health outcomes
Motivational interviewing can facilitate the trialogue between physicians, patients and care partners5-7
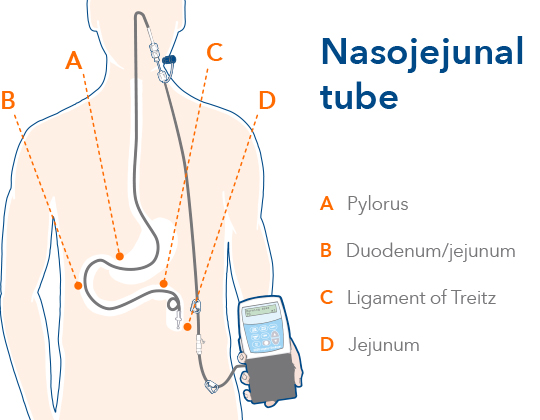
Testing response to Duodopa before surgery
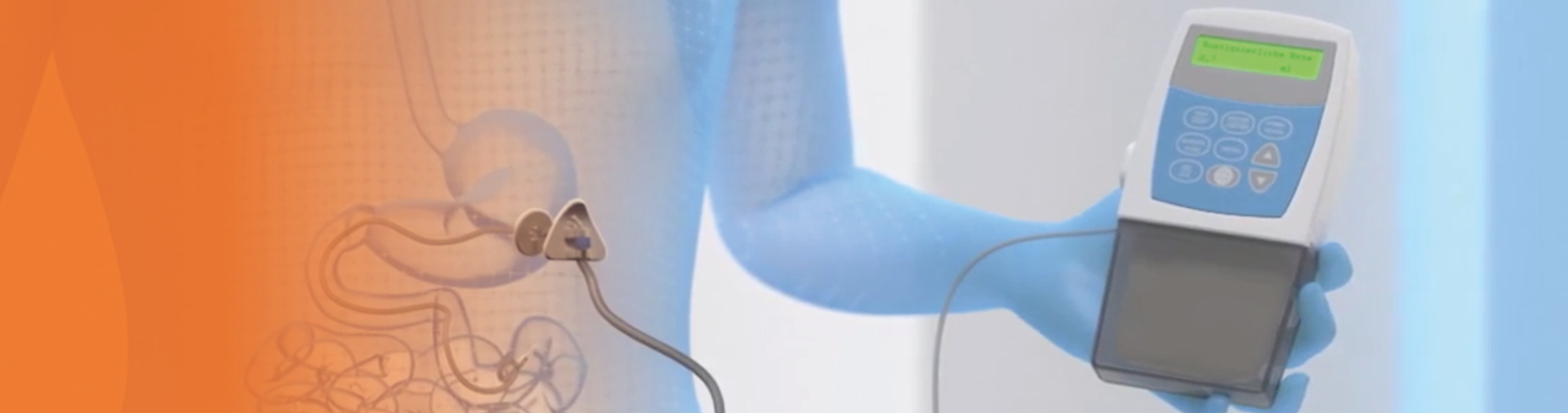
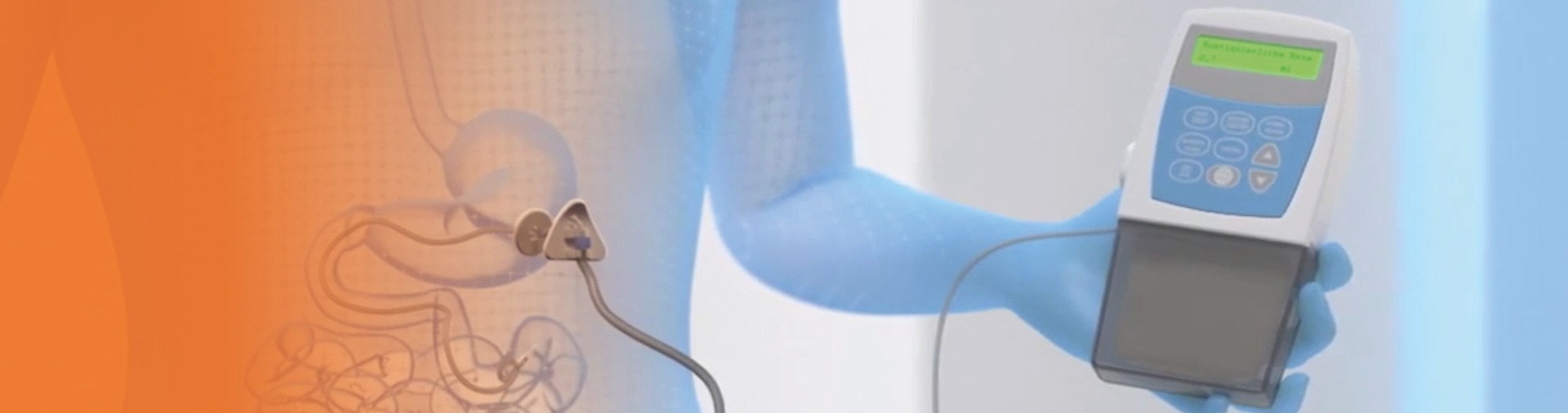
A temporary nasojejunal (NJ) tube could be considered, before a percutaneous endoscopic gastrostomy with a jejunal (PEG-J) tube
is placed, to determine whether the patient responds favorably to continuous drug delivery with Duodopa1
The NJ tube delivers Duodopa directly to the jejunum, and this test phase may take approximately 5 days4
In a prospective study, 91.5% of patients who completed the NJ test phase progressed to PEG-J tube placement8
In cases where the physician considers this assessment is not
necessary, the NJ test phase may be waived and treatment initiated
directly with placement of the PEG-J1
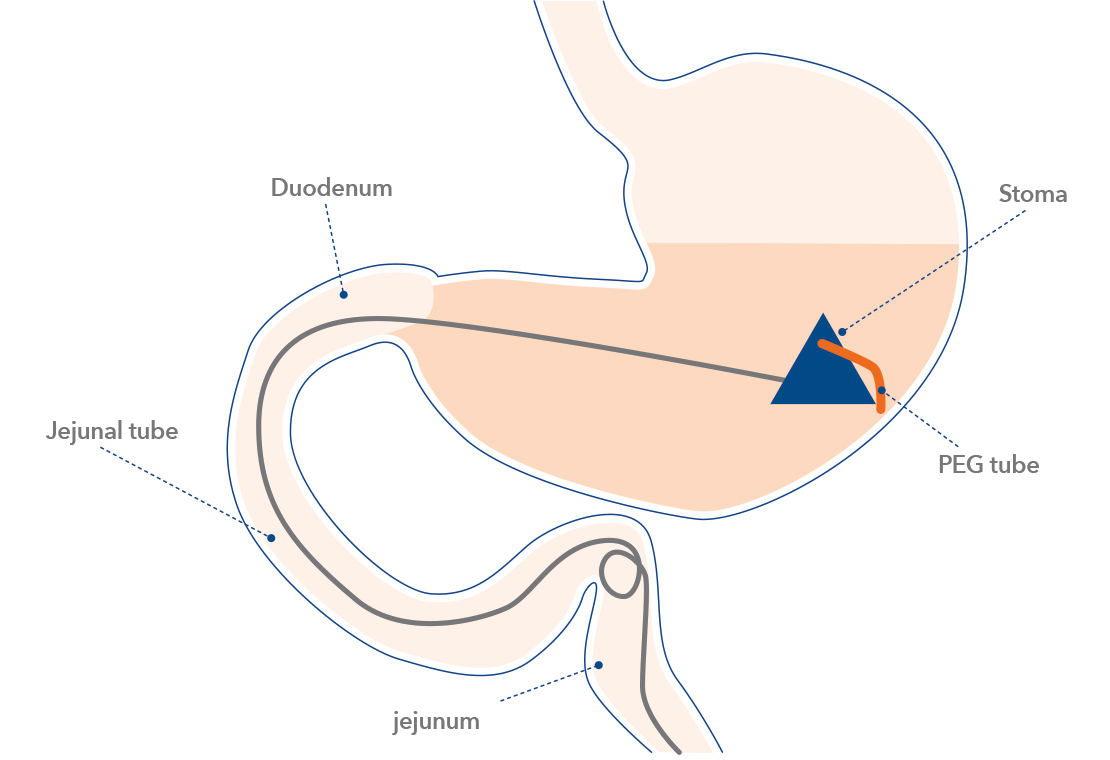
Overview of the PEG-J procedure
Initiating Duodopa therapy with the PEG-J procedure can be divided into five key steps:
1. Preparation
of the patient, including oral hygiene, antibiotic prophylaxis per institutional protocol, and placing them in a supine position9
2. Placing the PEG tube
by identifying an appropriate puncture site, puncturing the stomach and inserting the puncture cannula into the stomach under
endoscopic control, inserting the guide thread, securing the PEG tube to the guide thread and pulling out through the abdominal wall
until the retention plate is in direct contact with the inner gastric wall, and securing the PEG tube9
3. Attaching the Y-connector
to the PEG tube10
4. Positioning the intestinal J tube
by inserting into the Y-connector10
5. Fixing the intestinal J tube to the PEG tube
– the pump is connected and Duodopa treatment can start9
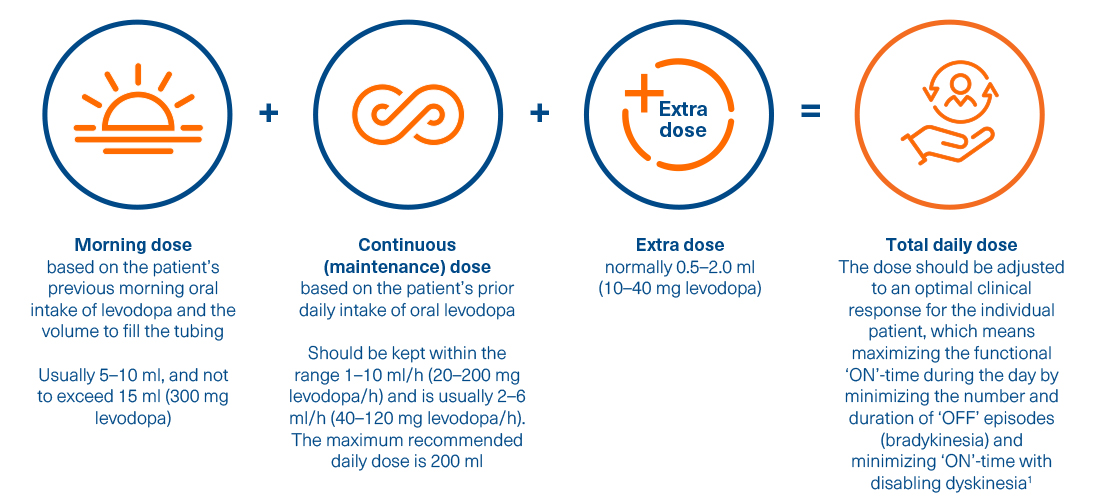
Calculating and titrating the Duodopa dose
The Duodopa dose is composed of three individually adjusted doses providing continuous treatment for up to 16 hours.1*
After initiation, fine adjustments of these three components of the overall dosage should be made over a few weeks to achieve an optimum dose1
* Treatment is usually administered during the patient's awake period. If medically justified, Duodopa may be administered for up to 24 hours1
ABBVIE CARE
[Placeholder for local affiliates to link to AbbVie Care services]
I want to receive more information via a product specialist
References
- Duodopa (levodopa/carbidopa intestinal gel) SmPC; [insert current data]
- Antonini A et al. Curr Med Res Opin 2018; 34(12):2063-2073.
* This study and publication were funded by AbbVie. - Van der Marck MA & Bloem BR. Parkinsonism Relat Disord 2014; Suppl. 1:S167-S173.
- Pedersen SW et al. Open Neurol 2012; 6:37-50.
- Bell RA et al. J Gen Intern Med 2002; 17(11):817-824.
- Markland D et al. J Social Clin Psychol 2005; 26(6):811-831.
- Street RL et al. Patient Educ Couns 2009; 74(3): 295-301.
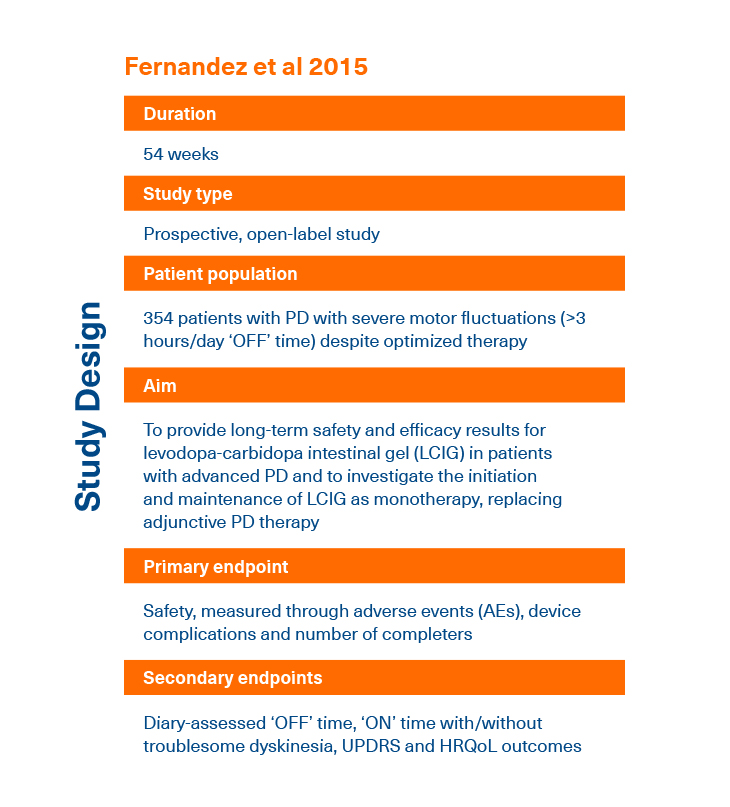
- Fernandez HH et al. Mov Disord 2015; 30:500-509.
- AbbVie PEG Kit 15 FR / 20 FR instructions for use.
- AbbVie J: Intestinal tube 9 FR for PEG 15 and 20 FR. Instructions for use.
- CADD-Legacy Duodopa Pump Model 1400. Patient Information.