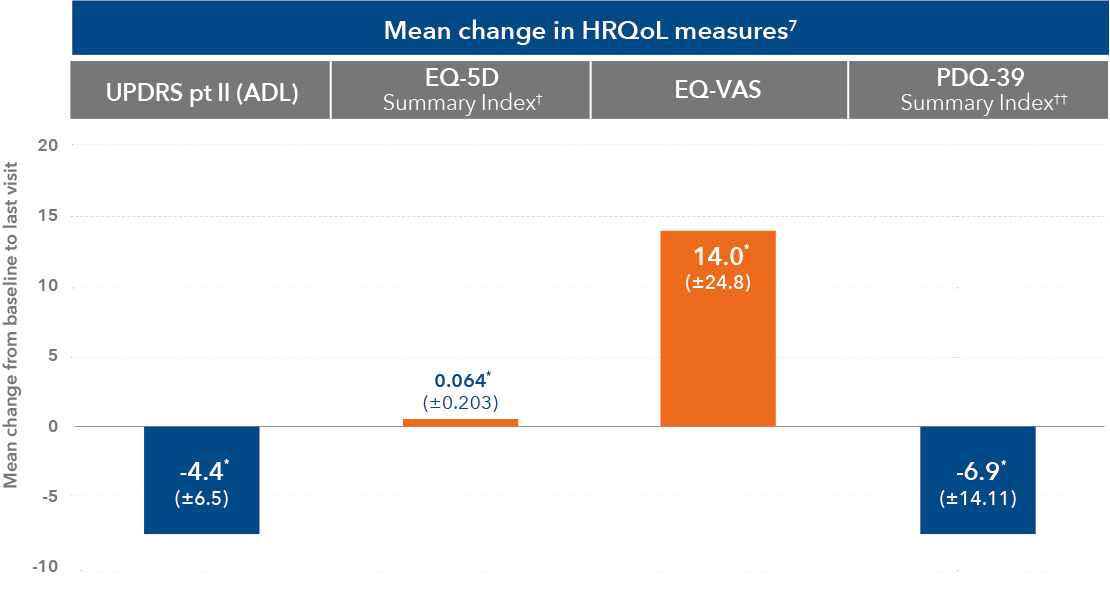Note to Affiliate: This document is based on the November 2023 Irish SmPC, the August 2020 J Tube IFU, the March 2019 NJ Tube IFU, the August 2020 PEG IFU, the 2019 CADD Duodopa Patient Information Guide, and the February 2019 CADD Duodopa Pump Operator's Manual.
Because ON TIME is their time
Duodopa is a device-aided therapy for advanced Parkinson’s disease:
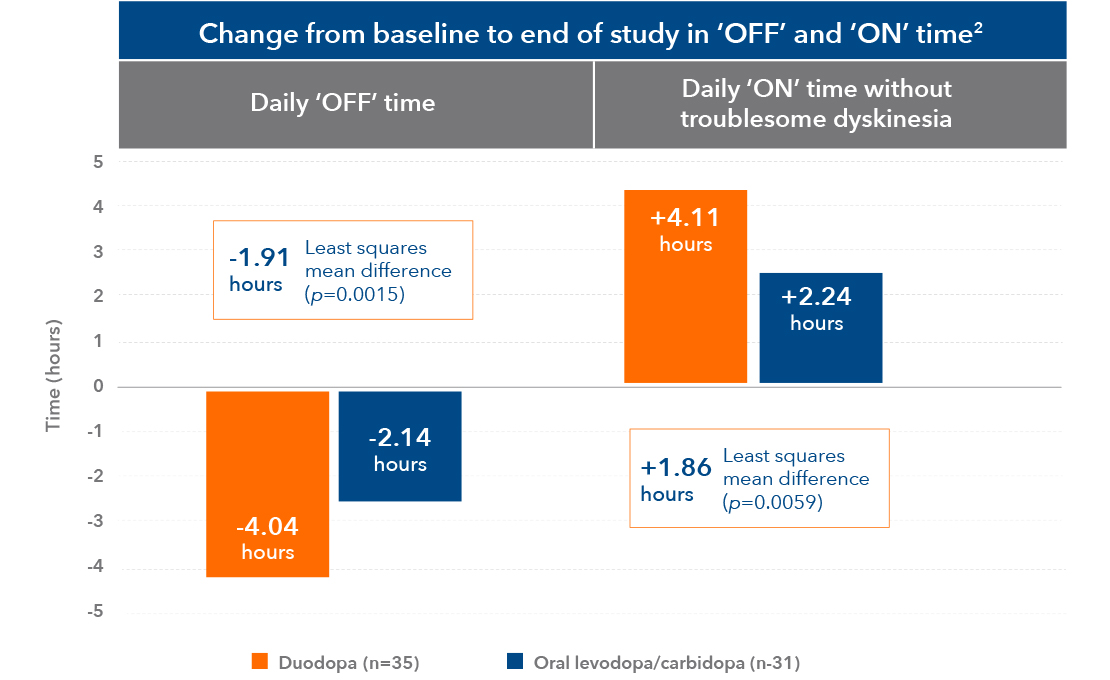
Improved ‘OFF’ and ‘ON’ time without troublesome dyskinesia2
Approximately 4 hours more ‘ON’ time each day – and 4 hours less ‘OFF’ time each day with Duodopa2
Most adverse events were related to the surgical procedure or the device, were mild to moderate in severity, occurred almost exclusively within the first week, and resolved in all cases.2
Most serious adverse events were due to the surgical procedure or device4
* Real-world evidence is collected outside of controlled clinical trials and has inherent limitations including a lesser ability for controlling factors
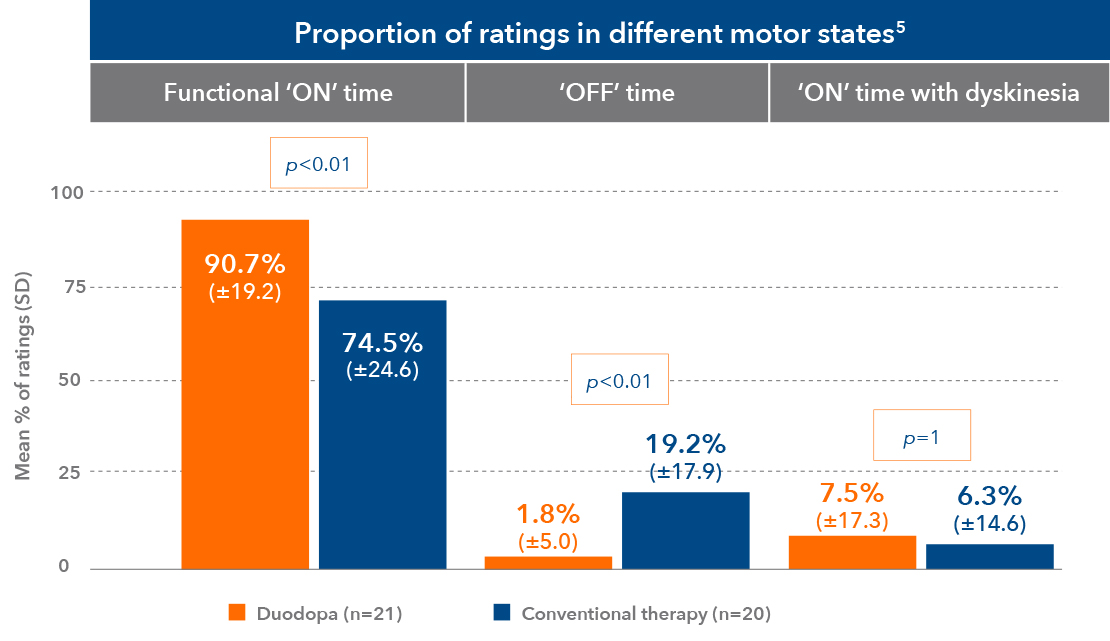
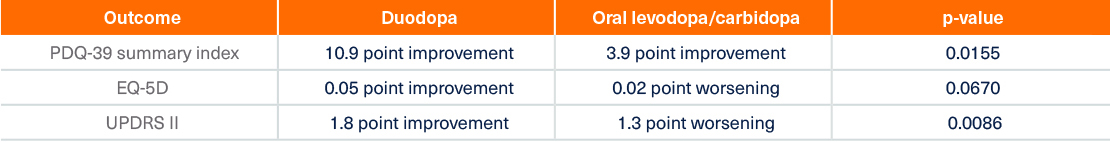
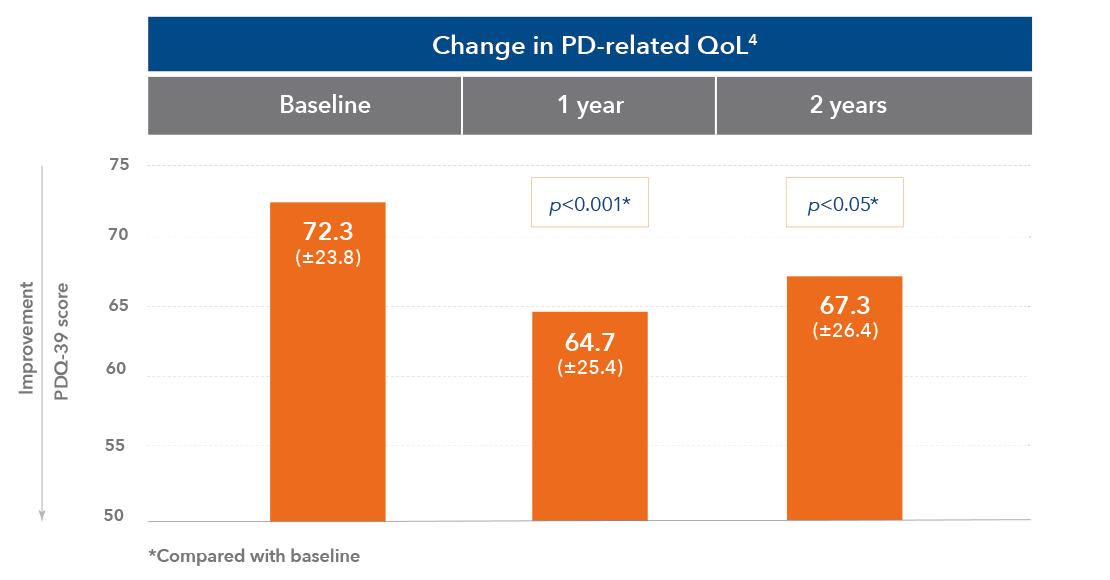
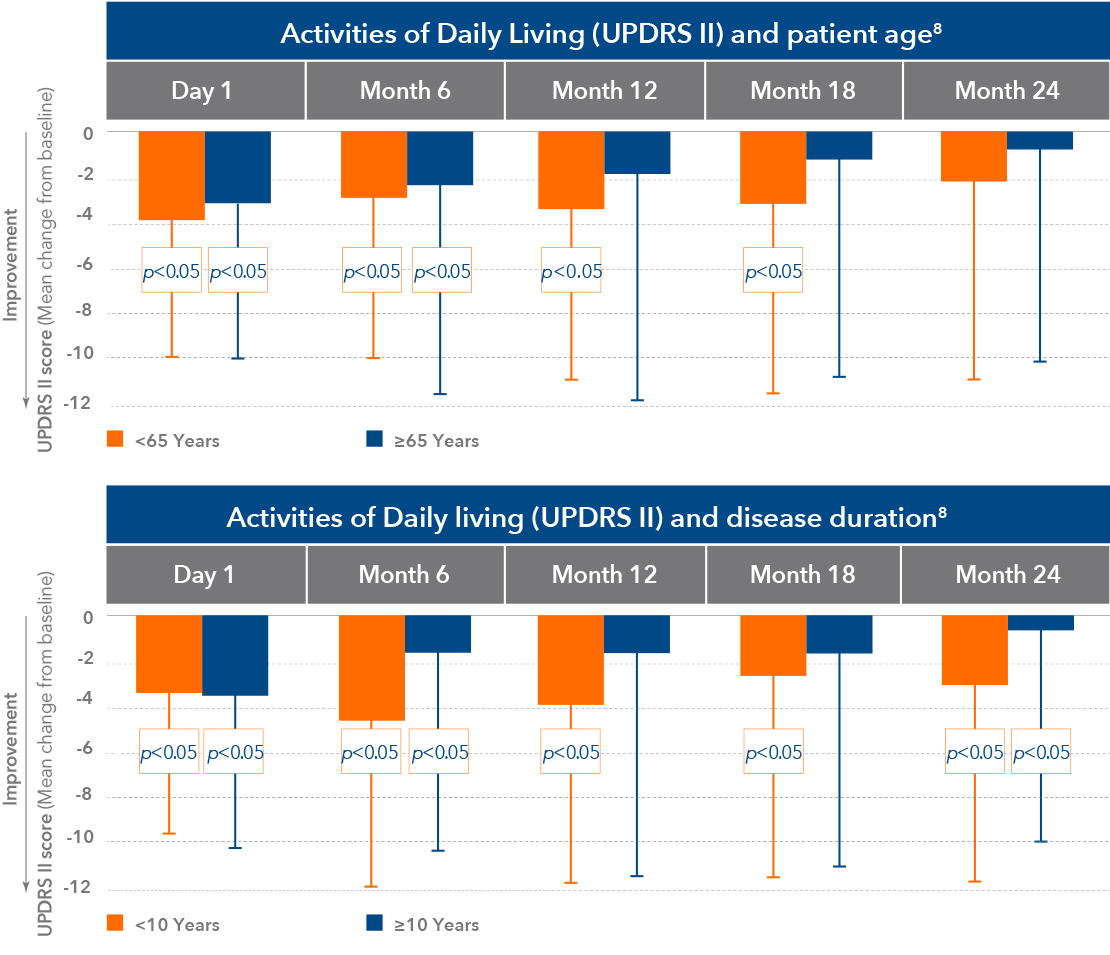
Duodopa offers significant improvements in QoL and ADL1,2,4,6
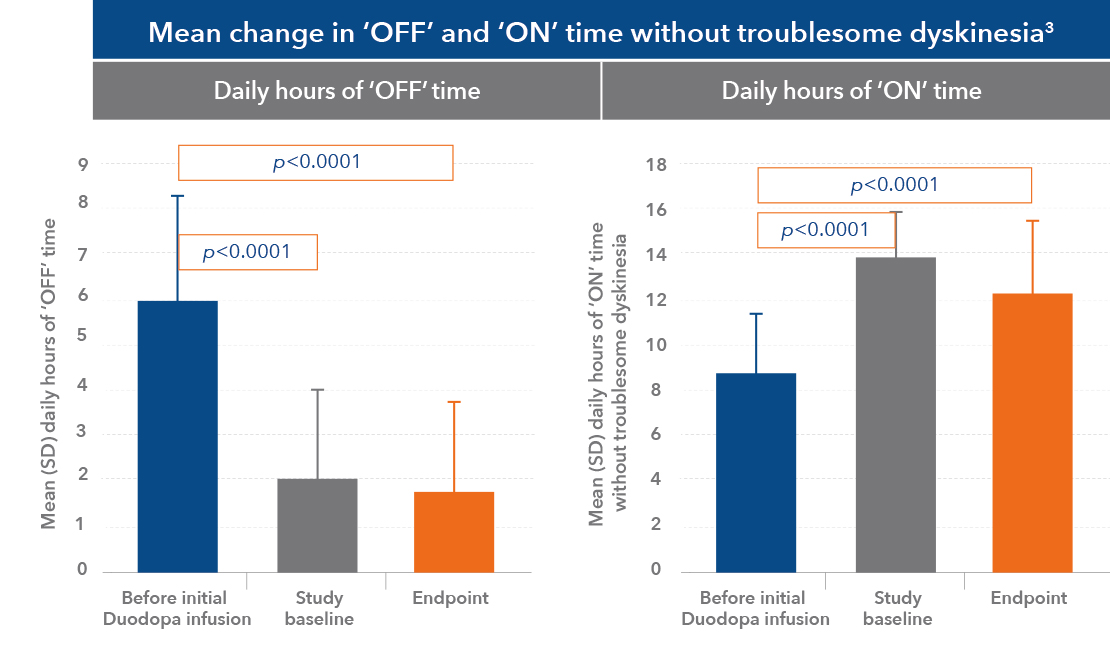
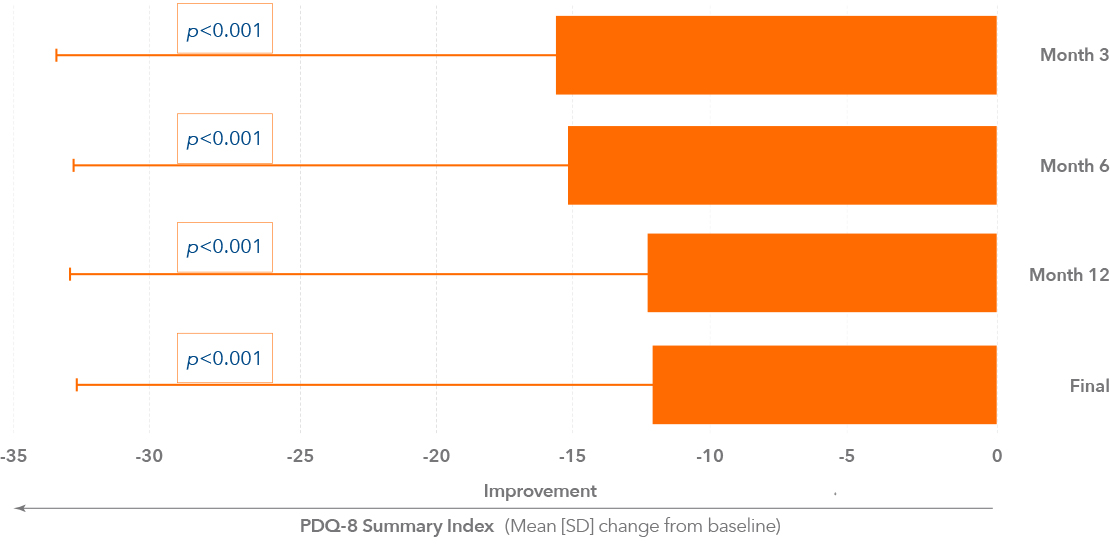
Quality of life improvements after 12 weeks of Duodopa therapy2
One-third of patients (32.8%) experienced an adverse event; 21.9% experienced a serious adverse event; 11.1% discontinued because of an adverse event; 12.7% experienced AE possibly related to LCIG; and 3.1% died during the study (cardiac failure and sudden death)6
* p<0.001 versus baseline, one-sample t test7
† In the pivotal study, EQ-5D Summary Index did not meet statistical significance based on the hierarchical testing procedure1
†† From screening to last visit. 7 of the 8 PDQ-39 domains (except social support) showed statistically significant mean improvement7
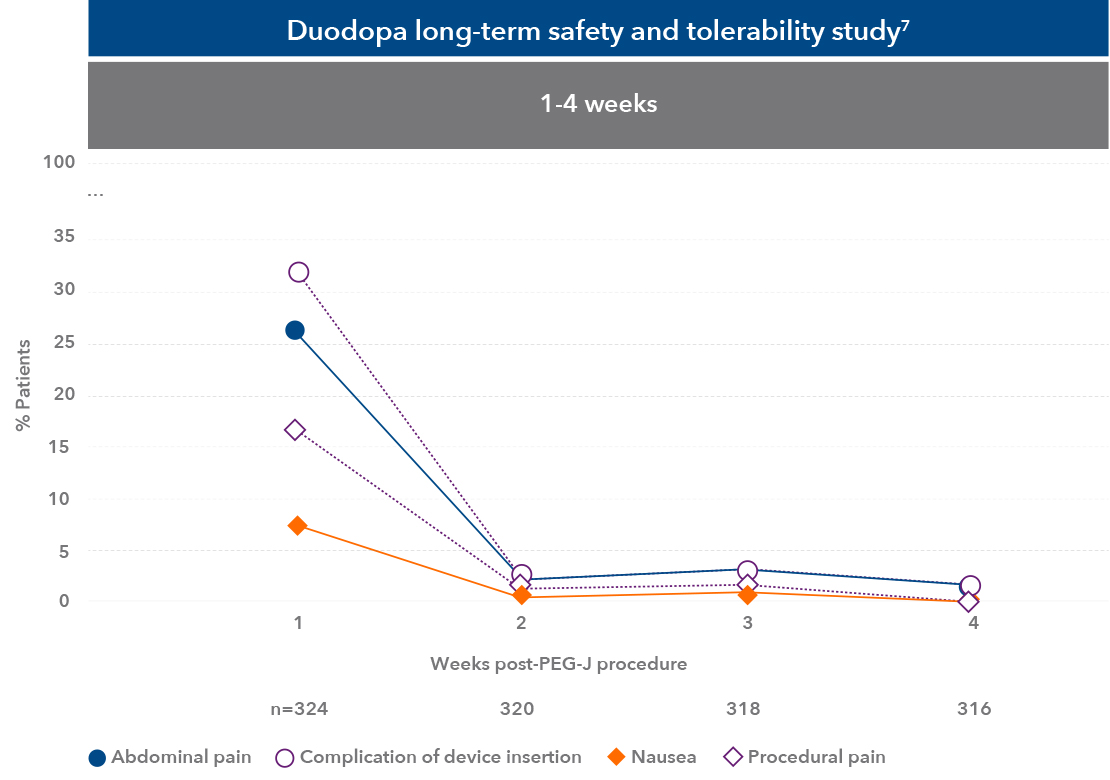
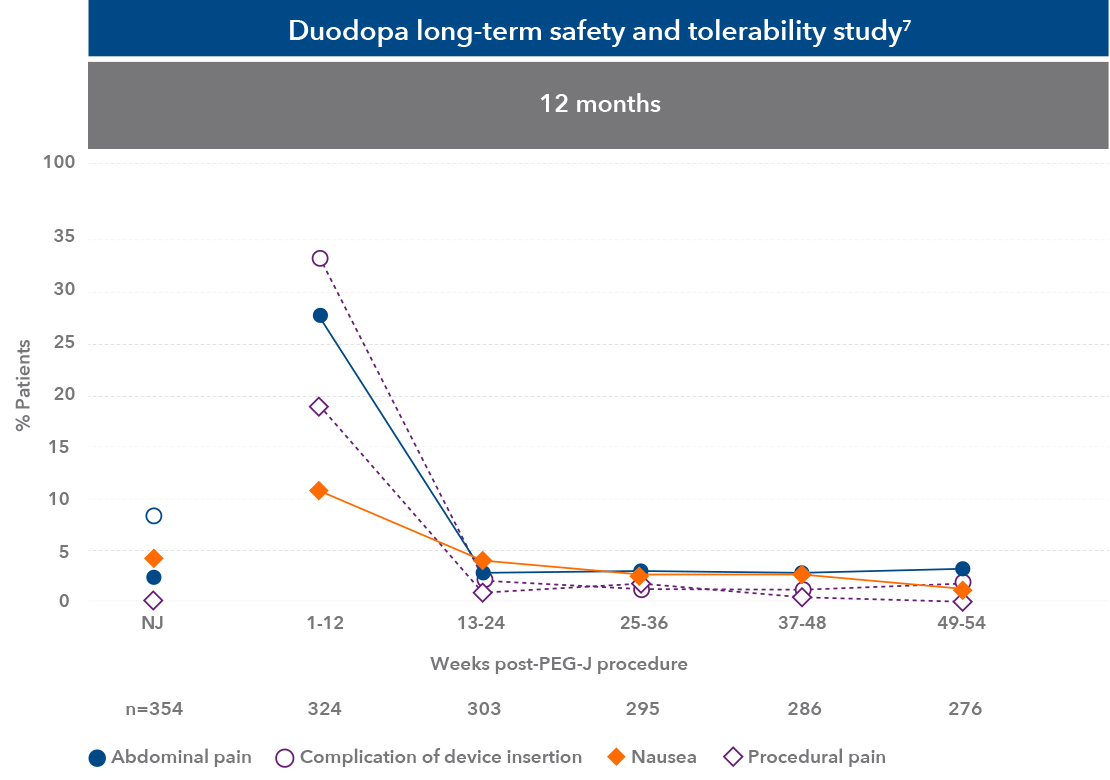
The most common adverse effects seen within the study were complication of device insertion, abdominal pain, and procedural pain7
Duodopa safety information7
Peak incidence of adverse reactions occurred in the first week after the PEG-J procedure, most were related to the surgical procedure, they resolved in most cases, and frequency reduced over time7
| • | Of 324 patients entering the PEG-J phase of a 54-week study, 8 patients discontinued due to a procedure-related or device-related adverse reaction7 |
A single event could be coded to >1 preferred term.
* Incidence of ARs peaked soon after the PEG-J procedure and reduced over time (≥10.0% during any time interval).1
NJ, nasojejunal tube test phase; n= number of patients
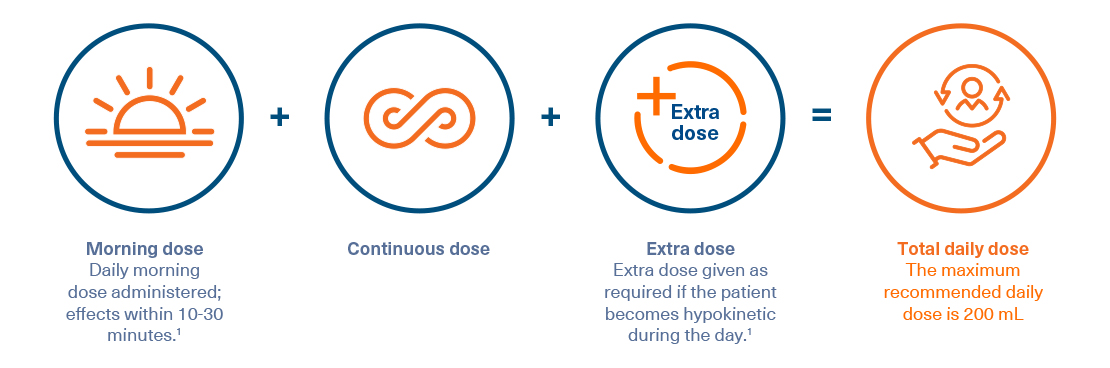
Duodopa dosing information1
- Replaces multiple oral doses, which can reduce pill burden and dosing inconvenience
- Intra-subject variability in levodopa plasma concentrations was lower for patients treated with Duodopa (21%) compared with patients treated with oral levodopa/carbidopa (67%)1
- Total Duodopa dose/day is composed of three individually adjusted doses: the morning bolus dose, the continuous maintenance dose, and extra bolus doses administered over approximately 16 hours1*
* Treatment is usually administered during the patient's awake period. If medically justified, Duodopa may be administered for up to 24 hours1
I want to receive more information via a product specialist
References
- Duodopa (levodopa/carbidopa intestinal gel) SmPC; [insert current data]
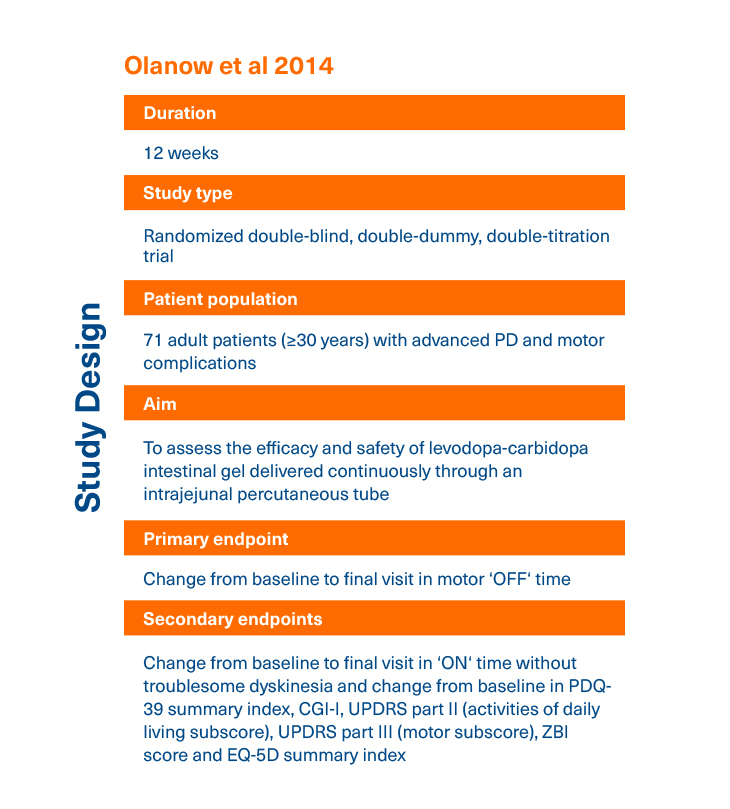
- Olanow CW et al. Lancet Neurol 2014;13(2):141-149.
- Fernandez HH et al. Mov Disord 2018; 33(6):928-936.
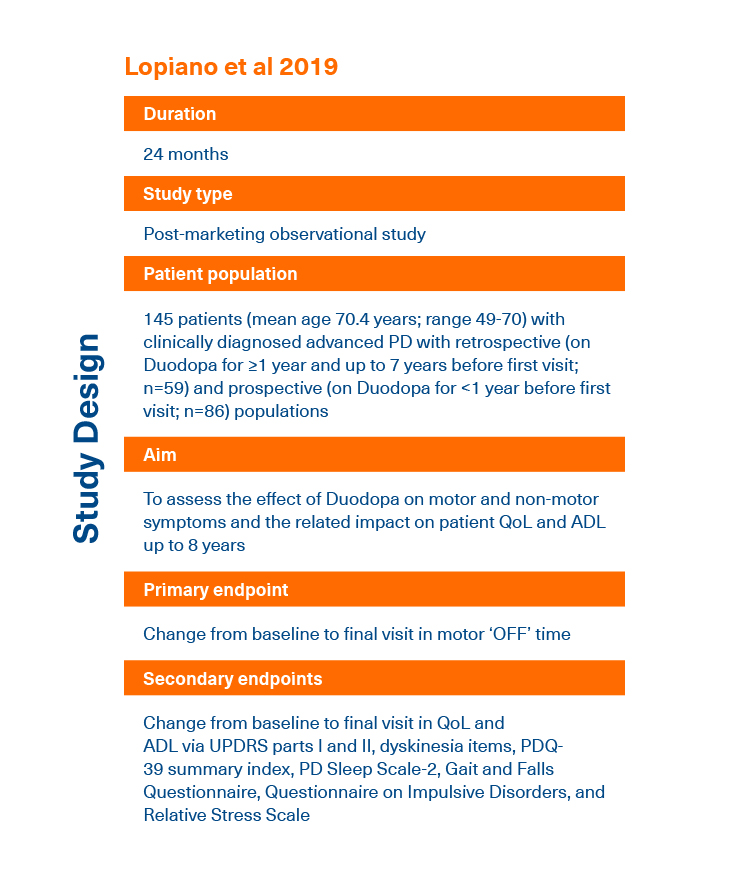
- Lopiano L et al. J Neurol 2019; 266:2164-2176.
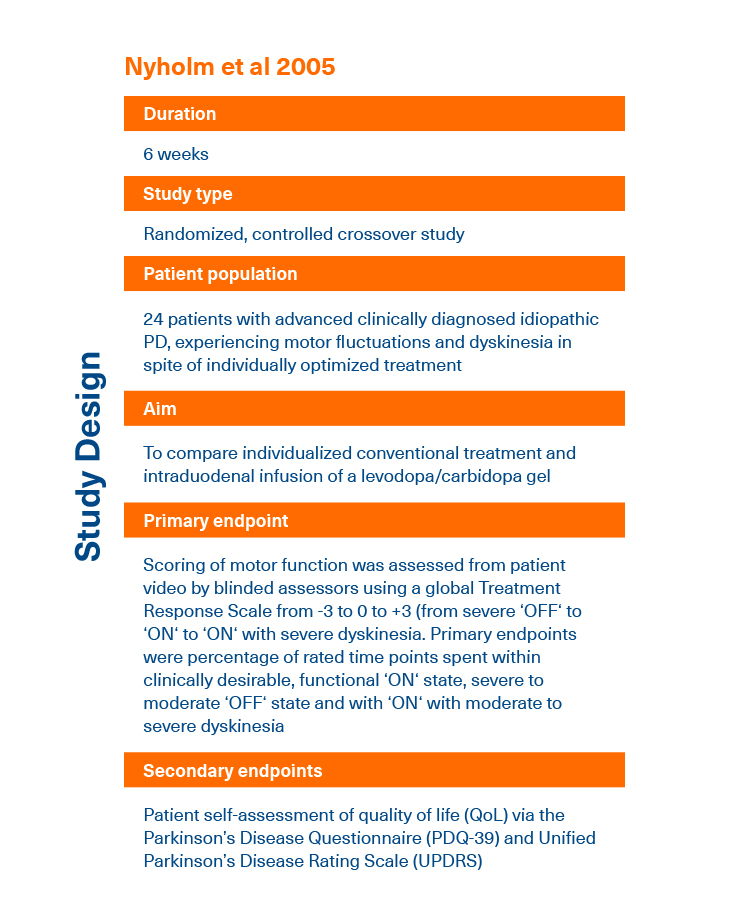
- Nyholm D et al. Neurology 2005; 64(2):216-223.
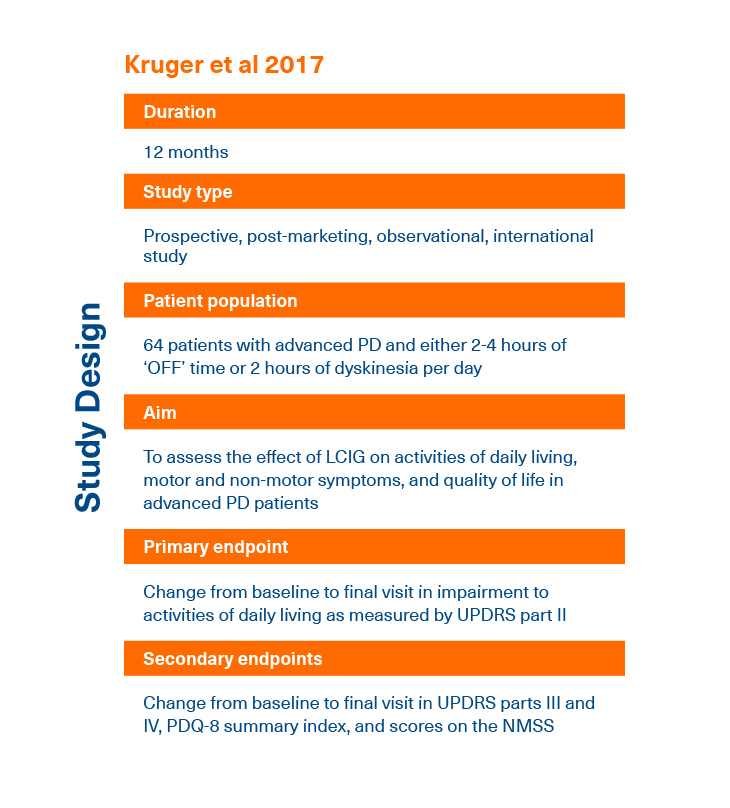
- Kruger R et al. Adv Ther 2017; 34(7):1741-1752.
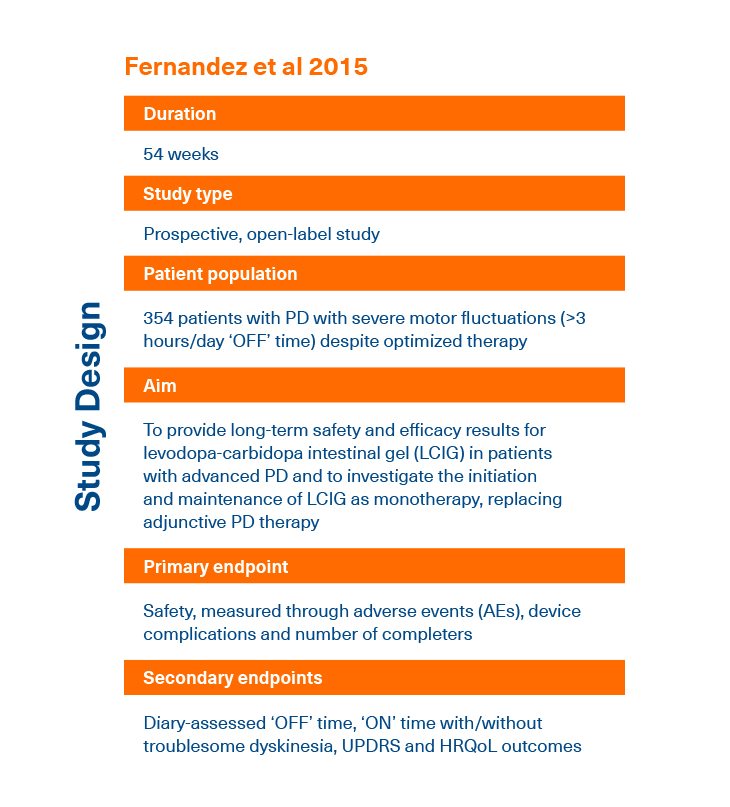
† This study and publication were funded by AbbVie. - Fernandez HH et al. Mov Disord 2015; 30:500-509.
- Antonini A et al. Neurodegen Dis Manag 2018; 8(3):161-170.
- Slevin JT et al. J Parkinson’s Dis 2015; 5(1):165-174.