Assessment scales and severity
There are numerous tools and scales available to assess the severity of atopic dermatitis (AD) as well as its impact on patients’ lives. Many are designed for use in the research setting rather than being appropriate for assessing patients in daily clinical practice.1,2 They include measures of the clinical signs, symptoms and disease control and tools designed to quantify the impact on patient quality of life.3-11
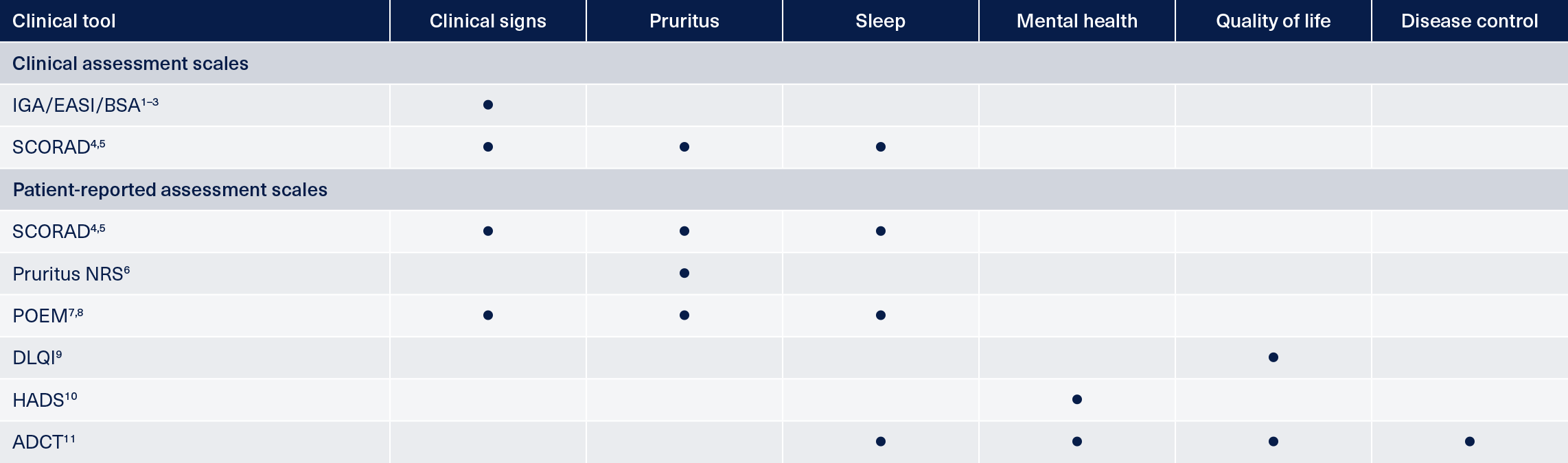
Examples of assessment tools in AD
AD: atopic dermatitis; ADCT: AD control tool; BSA: body surface area; DLQI: Dermatology Life Quality Index; EASI: Eczema and Severity Index; HADS: Hospital Anxiety and Depression Scale; IGA: investigator’s global assessment; NRS: numeric rating scale; POEM: Patient-Oriented Eczema Measure; SCORAD: SCORing Atopic Dermatitis.
No single assessment tool captures all aspects of AD, and there is currently no gold standard to assess objective disease severity in AD, so different tools are used in different situations.12 The number and variety of these tools can make it difficult to know which to use in the clinic and lead to inconsistent or infrequent uptake.12 To combat this, the Harmonising Outcome Measures for Eczema (HOME) initiative has been set up, aiming to standardize a core set of outcomes in AD that should be assessed in routine clinical practice.13,14
The HOME recommended assessment tools
HOME recommend different sets of tools for use in clinical trials and clinical practice. For measuring outcomes in clinical trials, they have identified four outcome domains that should be measured and reported in all clinical trials alongside the preferred tools for doing so:13
For assessing patients in clinical practice HOME have put together a list of suitable instruments for measuring a wider range of health domains than in clinical trials.14,15 These have been assessed based on their measurement properties and feasibility for use in clinical practice and allow for physicians to choose their preferred tool within any given domain.14,15
Currently, there are two domains where suitable tools have been identified and listed:14,15
- Patient reported symptoms – PO-SCORAD, POEM, Peak 24-hr NRS, PROMIS Itch questionnaire
- Disease control – RECAP, ADCT
Work is currently still ongoing to reach consensus on the preferred or suitable tools for measuring physician global assessment, clinician reported signs and eczema-specific quality of life in AD.14
A quick overview of the scales
Investigator’s Global Assessment (IGA) and Eczema Area and Severity Index (EASI) are scoring systems that grade the physical signs of atopic dermatitis/eczema.1,2 The validated Investigator’s Global Assessment scale for AD (vIGA-AD™) was developed to provide a validated, standardized form of the IGA for use in AD clinical trials.1 Both score the clinical appearance of AD skin based on a set of defined criteria including the presence of absence of erythema, edema/papulation and lichenification.1,2 The EASI score also takes into account the presence and extent of excoriation as well as the proportion of body surface area affected while the validated IGA-AD includes the presence of oozing or crusts in AD skin.1,2
EASI is measured on a scale of 0 to 72 where a score of 0 equals clear skin, 0.1‑1.0 indicates almost clear skin, 1.1‑7.0 mild disease, 7.1‑21.0 moderate disease, 21.1‑50.0 severe disease and 50.1‑72.0 very severe disease.16
The vIGA-AD score is on a scale of 0 to 4 with 0 representing clear skin and 4 severe disease and the score is selected using the descriptors that best describe the overall appearance of lesions at a given time point.1
A clinician and patient-reported measure of signs and symptoms, the Scoring Atopic Dermatitis (SCORAD) index combines scores for the extent and severity of disease as well as subjective symptoms.4,5 It takes into account the intensity and distribution of five different skin characteristics; erythema; edema/papulation; oozing/crusts; excoriations; lichenification, plus skin dryness on non-lesional areas of the body. Subjective symptoms of pruritus and sleep loss are also factored into the calculation.4,5
As part of their Clinical Practice Set, HOME recommend the use of the PO-SCORAD measure which is available to patients as a downloadable mobile app.13
Itch severity measured on a range or scale in which a patient selects a value corresponding to the intensity of itch over a given timescale. Usually on a scale of 0 (no itch) to 10 (worst imaginable itch).6
Different NRS definitions are used including:17
- Peak NRS score over 24 hours or 7 days
- Average NRS score over 24 hours or 7 days
- Average peak NRS score over 7 days
Some clinical studies also assess the proportion of patients reaching the minimal clinically important difference (MCID), usually defined as a ≥4-point improvement in score.18
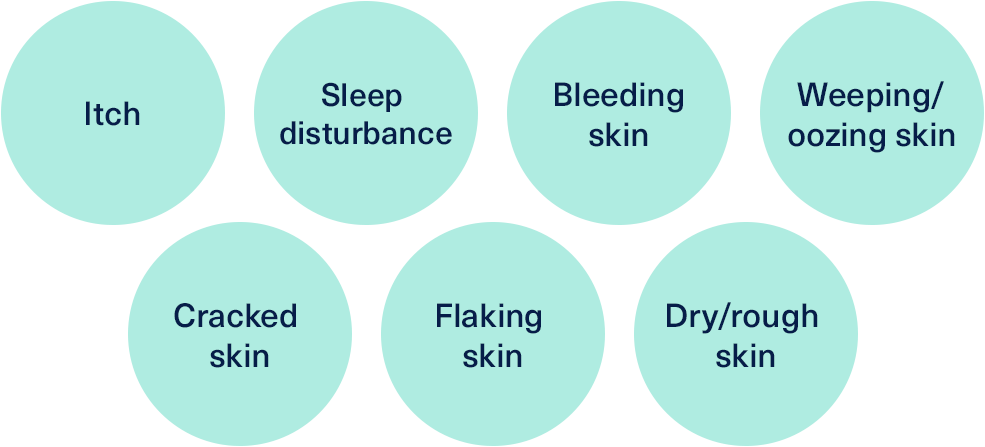
The Patient-Oriented Eczema Measure (POEM) assessment tool is a questionnaire format that asks patients to rate the frequency of skin-related symptoms and interrupted sleep over the previous week.19,20 Each of seven symptoms is then assigned a score from 0-4 which are added together to produce the overall POEM score. A score of 0-2 denotes clear skin, 3-7 mild AD, 8-16 moderate disease, 17-24 severe disease and 25-28 very severe disease.19,20
The symptoms included in the measure are:19,20
The Dermatology Life Quality Index (DLQI) measure is specifically designed to assess patients’ perception of how their skin condition impacts their quality of life. It is designed for use in daily clinical practice and covers multiple domains including pain, ability to carry out or participate in everyday activities and the effect on patients’ confidence and personal relationships.21
The DLQI is designed for use in adult patients, alternative quality of life measures have been developed for use in children and infants.21
The Hospital Anxiety and Depression Scale (HADS) specifically assesses the impact of disease on mental health.22 It is a questionnaire-based tool that asks patients to assess how they have been feeling over the previous week against various parameters such as tension, enjoyment and relaxation.
The answers given are used to calculate a score which indicates the relative likelihood of depression or anxiety.22,23
Both the Recap of Atopic Dermatitis (RECAP) and Atopic Dermatitis Control Tool (ADCT) are similar measures for the assessment of disease control in atopic dermatitis.24,25 Both are short, validated questionnaires that assess the severity and frequency of symptoms such as itch and the impact of AD on sleep, daily activities and mood.24,25
They are designed to give an overview of the severity of disease over the preceding week.
REFERENCES
- Simpson E, et al. The Validated Investigator Global Assessment for Atopic Dermatitis (vIGA-AD): The development and reliability testing of a novel clinical outcome measurement instrument for the severity of atopic dermatitis. J Am Acad Dermatol 2020;83:839–46.
- Hanifin JM, et al. The eczema area and severity index (EASI): assessment of reliability in atopic dermatitis. EASI Evaluator Group. Exp Dermatol. 2001;10:11–8.
- Chopra R, et al. Severity strata for Eczema Area and Severity Index (EASI), modified EASI, Scoring Atopic Dermatitis (SCORAD), objective SCORAD, Atopic Dermatitis Severity Index and body surface area in adolescents and adults with atopic dermatitis. Br J Dermatol. 2017;177:1316–21.
- European Task Force on Atopic Dermatitis. Severity Scoring of Atopic Dermatitis: The SCORAD Index. Dermatology. 1993;186:23–31.
- Oranje AP, et al. Practical issues on interpretation of scoring atopic dermatitis: the SCORAD index, objective SCORAD and the three-item severity score. Br J Dermatol. 2007;157:645–8.
- Yosipovitch G, et al. Peak Pruritus Numerical Rating Scale: psychometric validation and responder definition for assessing itch in moderate-to-severe atopic dermatitis. Br J Dermatol. 2019;181:761–9.
- Schram ME, et al. EASI, (objective) SCORAD and POEM for atopic eczema: responsiveness and minimal clinically important difference. Allergy 2012;67:99–106.
- Howells L, et al. How should minimally important change scores for the Patient-Oriented Eczema Measure be interpreted? A validation using varied methods. Br J Dermatol. 2018;178:1135–42.
- Basra MK, et al. Determining the minimal clinically important difference and responsiveness of the Dermatology Life Quality Index (DLQI): further data. Dermatology. 2015;230:27–33.
- Bjelland I, et al. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. 2002;52:69–77.
- Simpson E, et al. Validation of the Atopic Dermatitis Control Tool (ADCT©) using a longitudinal survey of biologic-treated patients with atopic dermatitis. BMC Dermatology. 2019;19:15.
- Boguniewicz M, et al. Expert Perspectives on Management of Moderate-to-Severe Atopic Dermatitis: A Multidisciplinary Consensus Addressing Current and Emerging Therapies. J Allergy Clin Immunol Pract. 2017;5:1519–31.
- Schmitt J, et al. Towards global consensus on outcome measures for atopic eczema research: results of the HOME II meeting. Allergy. 2012;67(9):1111–7.
- Harmonizing Outcomes for Eczema (HOME). Clinical practice set. Available at http://www.homeforeczema.org/ clinical-practice-set.aspx. Accessed February 2022.
- Lesham YA, et al. Measuring atopic eczema symptoms in clinical practice: The first consensus statement from the Harmonising Outcome Measures for Eczema in clinical practice initiative. J Am Acad Dermatol. 2020;82(5):1181–1186.
- Lesham YA, et al. What the Eczema Area and Severity Index score tells us about the severity of atopic dermatitis: an interpretability study. Br J Dermatol. 2015;172(5):1353–7.
- de Wijs LEM, et al. Effectiveness of dupilumab treatment in 95 patients with atopic dermatitis: daily practice data. Br J Dermatol. 2020;182:418–26.
- Silverberg JI, et al. Upadacitinib plus topical corticosteroids in atopic dermatitis: Week 52 AD Up study results. J Allergy Clin Immunol. 2021;S0091–6749(21)01212−4.
- Charman CR, et al. The patient-oriented eczema measure: development and initial validation of a new tool for measuring atopic eczema severity from the patients’ perspective. Arch Dermatol. 2004;140:1513–19.
- University of Nottingham. POEM for self completion and/or proxy completion. Available at: https://www.nottingham.ac.uk/ research/groups/cebd/documents/ methodological-resources/poem-for-self-completion-or-proxy-completion.pdf. Accessed February 2022.
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)--a simple practical measure for routine clinical use. Clin Exp Dermatol 1994;19:210–16.
- Hospital Anxiety and Depression Scale (HADS). Available at https://www.svri.org/sites/default/ files/attachments/2016-01-13/HADS.pdf. Accessed February 2022.
- Stern AF. The hospital anxiety and depression scale. Occup Med 2014;64:393–4.
- University of Nottingham. Centre of Evidence Based Dermatology Resources. RECAP. Available at: https://www.nottingham.ac.uk/ research/groups/cebd/resources/ recap.aspx. Accessed February 2022.
- Atopic Dermatitis Control Tool. About ADCT. Available at: https://www.adcontroltool.com/ about-adct1. Accessed February 2022.