RA integrated safety analysis2,3
- Included data from 6 randomized controlled Phase 3 RINVOQ RA trials.
- Patients who switched from placebo, adalimumab, MTX, or abatacept to RINVOQ were included in the RINVOQ analysis set from the start of RINVOQ, while those who switched from RINVOQ to adalimumab were included in the adalimumab analysis set from the start of adalimumab and were censored at the time of switch. MTX monotherapy was censored at time of rescue to combination therapy (addition of RINVOQ).
- Only data from treatment groups with long-term exposures were analyzed; therefore, data for placebo or abatacept (which were only available for the short-term, double-blind controlled periods) were not included.
- Treatment-emergent adverse events were defined as any AE with an onset date on or after the first dose of study drug and ≤30 days (≤70 days for adalimumab) after the last dose of study drug.
- TEAEs were reported as exposure-adjusted adverse event rates (EAERs; E/100 PY), which included both incident and recurrent events up to a cutoff date of June 30, 2020.
Integrated safety data set from the RINVOQ Phase 3 program in RA3
- MTX monotherapy (N=314) (1 trial): SELECT-EARLY (MTX-naive)
- Adalimumab 40 mg EOW (N=579) (1 trial): SELECT-COMPARE (MTX-IR)
- RINVOQ 15 mg QD (N=3209) (6 trials): SELECT-EARLY (MTX-naive), SELECT-NEXT (csDMARD-IR), SELECT-MONOTHERAPY (MTX-IR), SELECT-COMPARE (MTX-IR), SELECT-BEYOND (bDMARD-IR), SELECT-CHOICE (bDMARD-IR)
- Upadacitinib 30 mg QD (N=1204) (4 trials): Upadacitinib 30 mg QD results not provided since not an approved dosage in RA.
PsA integrated safety analysis4,5
- Included data from 2 randomized controlled Phase 3 RINVOQ PsA trials.
- Stable background treatment with ≤2 nonbiologic DMARDs was permitted; background therapy was not required.
- At Week 24, all patients randomized to placebo were switched to RINVOQ 15 mg or upadacitinib 30 mg QD in a blinded manner.
- Only data from treatment groups with long-term exposures were analyzed, therefore, data for placebo (which were only available for the short-term, double-blind controlled period) were not included.
- Treatment-emergent adverse events were defined as any AE with an onset date on or after the first dose and ≤30 days (≤70 days for adalimumab) after the last dose of study drug.
- TEAEs were reported as exposure-adjusted adverse event rates (EAERs; E/100 PY) up to a cutoff date of June 20, 2020.
Integrated safety data set from the RINVOQ Phase 3 program in PsA5
- Adalimumab 40 mg EOW (N=429) (1 trial): SELECT-PsA 1 (nonbiologic DMARD-IR)
- RINVOQ 15 mg QD (N=640) (2 trials): SELECT-PsA 1 (nonbiologic DMARD-IR), SELECT-PsA 2 (biologic DMARD-IR)
- Upadacitinib 30 mg QD (N=641) (2 trials): Upadacitinib 30 mg QD results not provided since not an approved dosage in PsA.
AS SELECT-AXIS 1 Phase 2/3 clinical study6
- Included patients with active AS and an inadequate response to conventional therapy.
- Permitted concomitant medications were csDMARDs (methotrexate, leflunomide, sulfasalazine, or hydroxychloroquine), oral glucocorticoids, NSAIDs, and analgesics.
- At Week 14, all patients randomized to placebo were switched to RINVOQ 15 mg QD.
- Only data from treatment groups with long-term exposures were analyzed, therefore, data for placebo (which were only available for the short-term, double-blind controlled period) were not included.
- Long-term safety reports are based on available data on cutoff date of January 31, 2020, and were assessed as rate of treatment-emergent AEs reported as E/100 PY.
- Treatment-emergent adverse events were defined as AEs that began or worsened in severity after the first dose and through 30 days after the last dose of study drug.
Safety data set from the RINVOQ Phase 2/3 study in AS6
- RINVOQ 15 mg QD (N=182) (1 trial): SELECT-AXIS 1 (NSAID-IR)
TEAEs observed with RINVOQ 15 mg in 3 separate safety data sets in RA, PsA, and AS
Select the RA, PsA, and AS tabs for full safety tables with comparator arms.
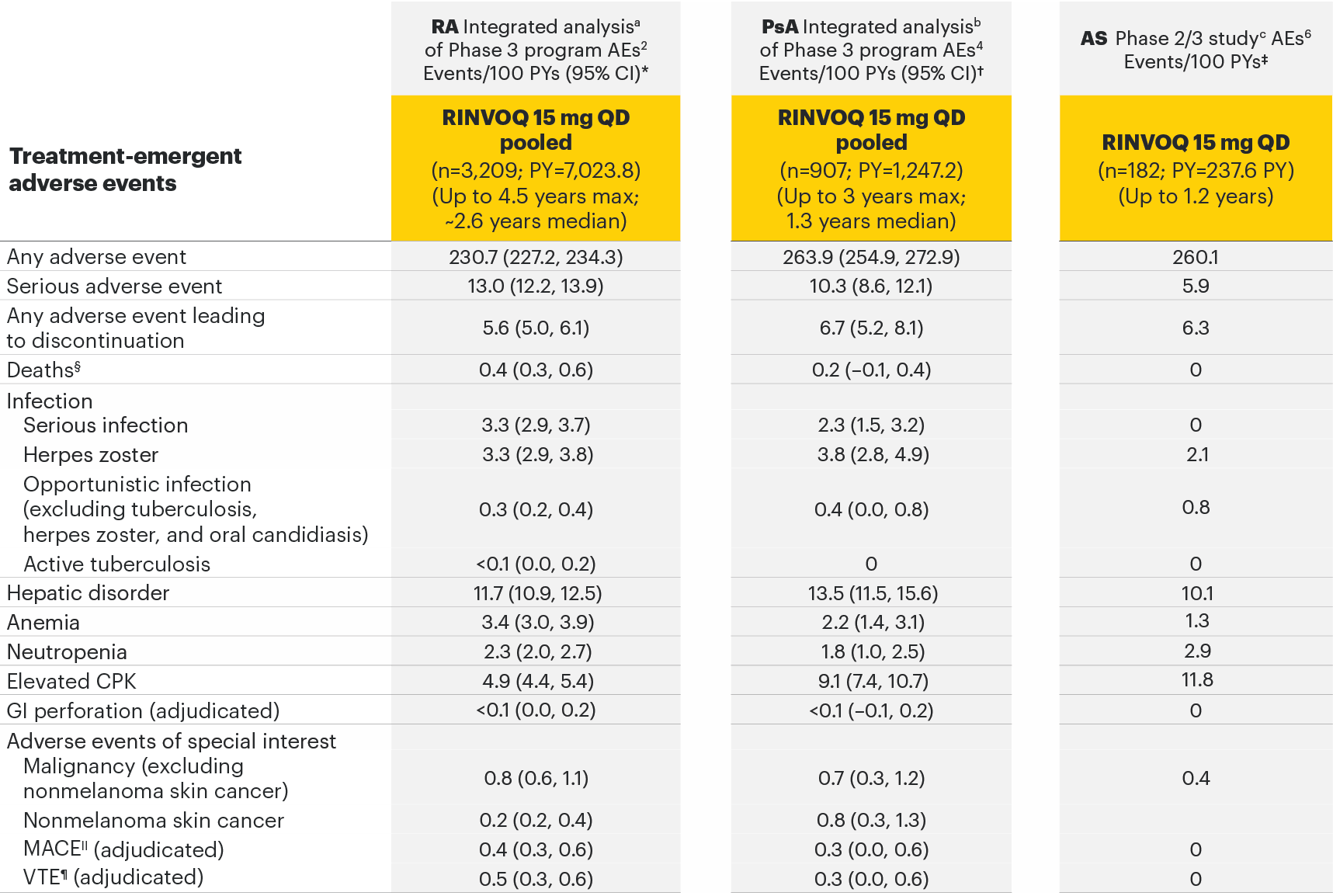
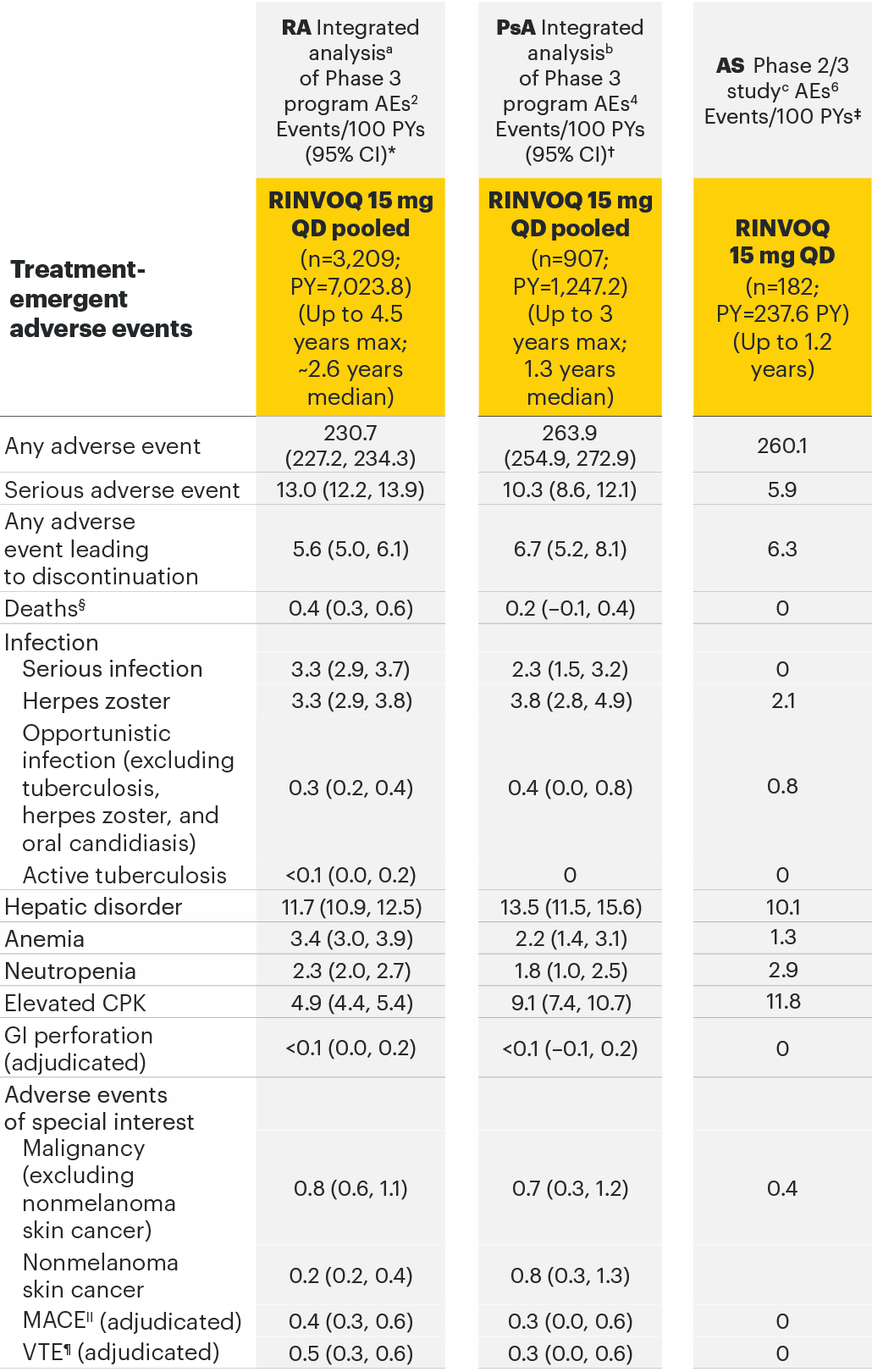
Adverse events observed in 3 separate safety analyses across rheumatology2-6
aSELECT-EARLY, SELECT-COMPARE, SELECT-MONOTHERAPY, SELECT-NEXT, SELECT-BEYOND, and SELECT-CHOICE.
bSELECT-PsA 1 and SELECT-PsA 2.
cSELECT-AXIS 1.
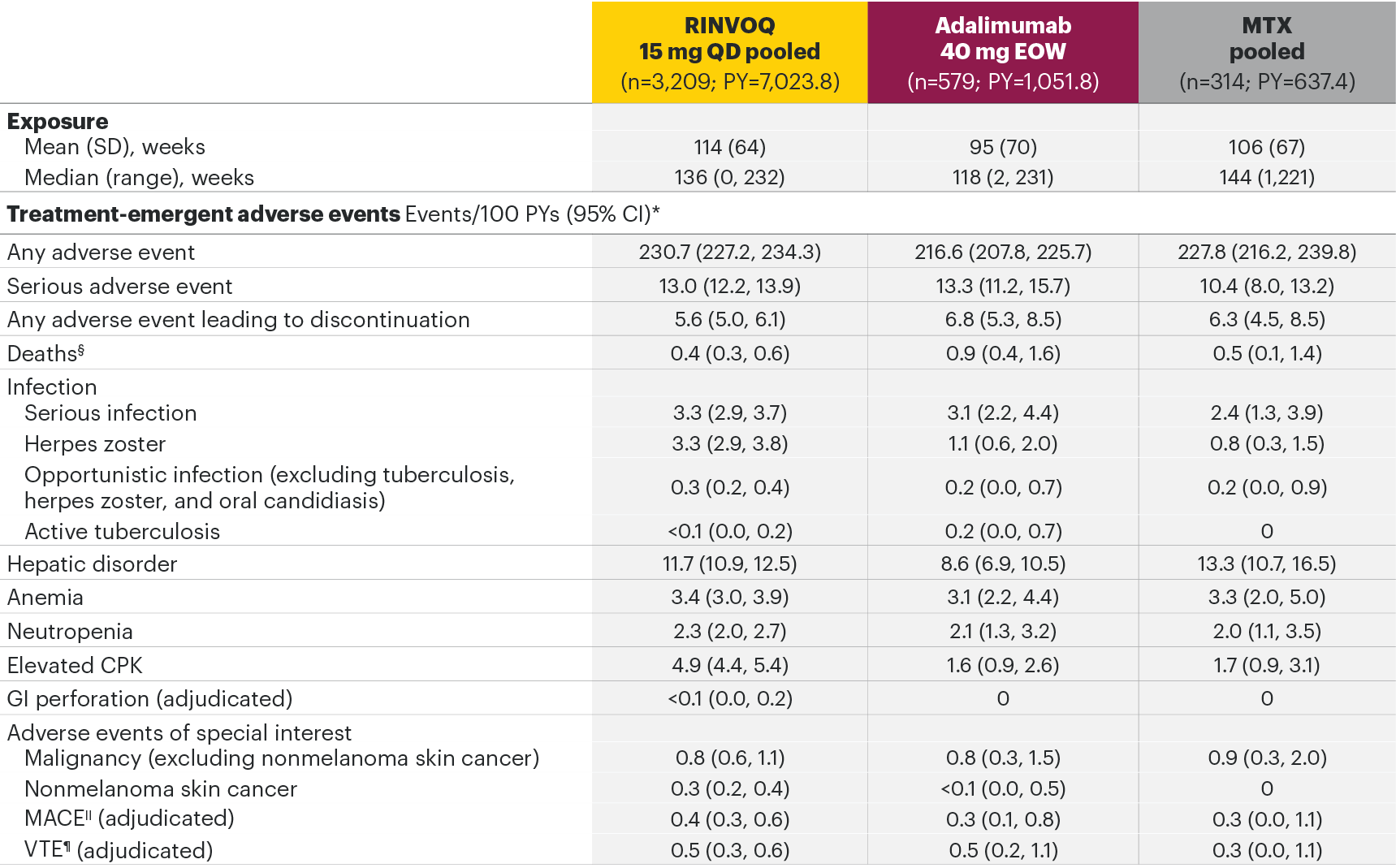
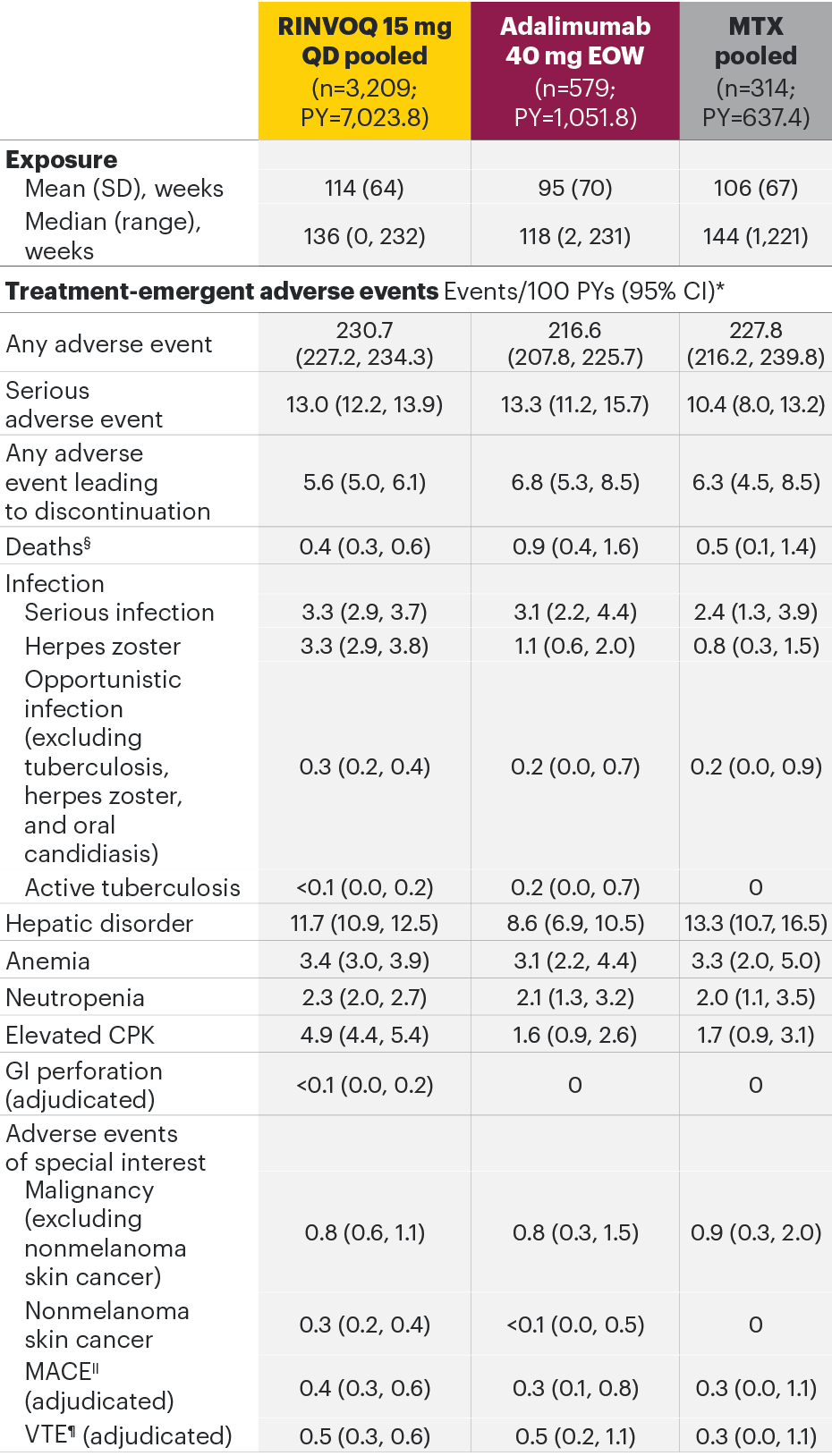
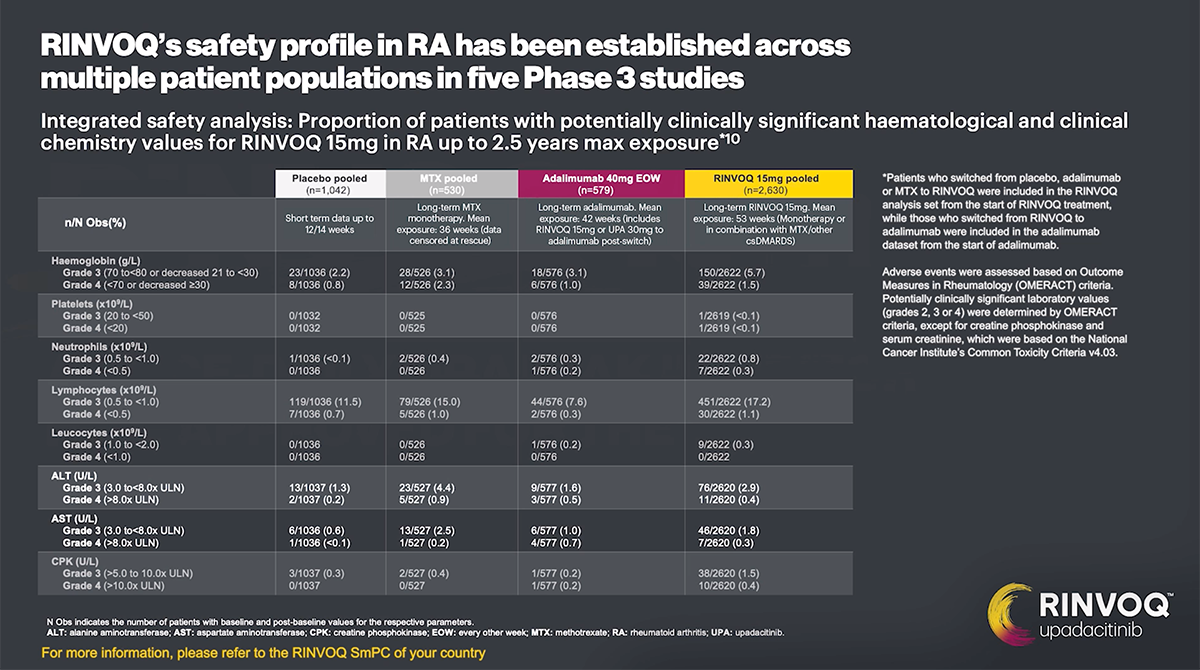
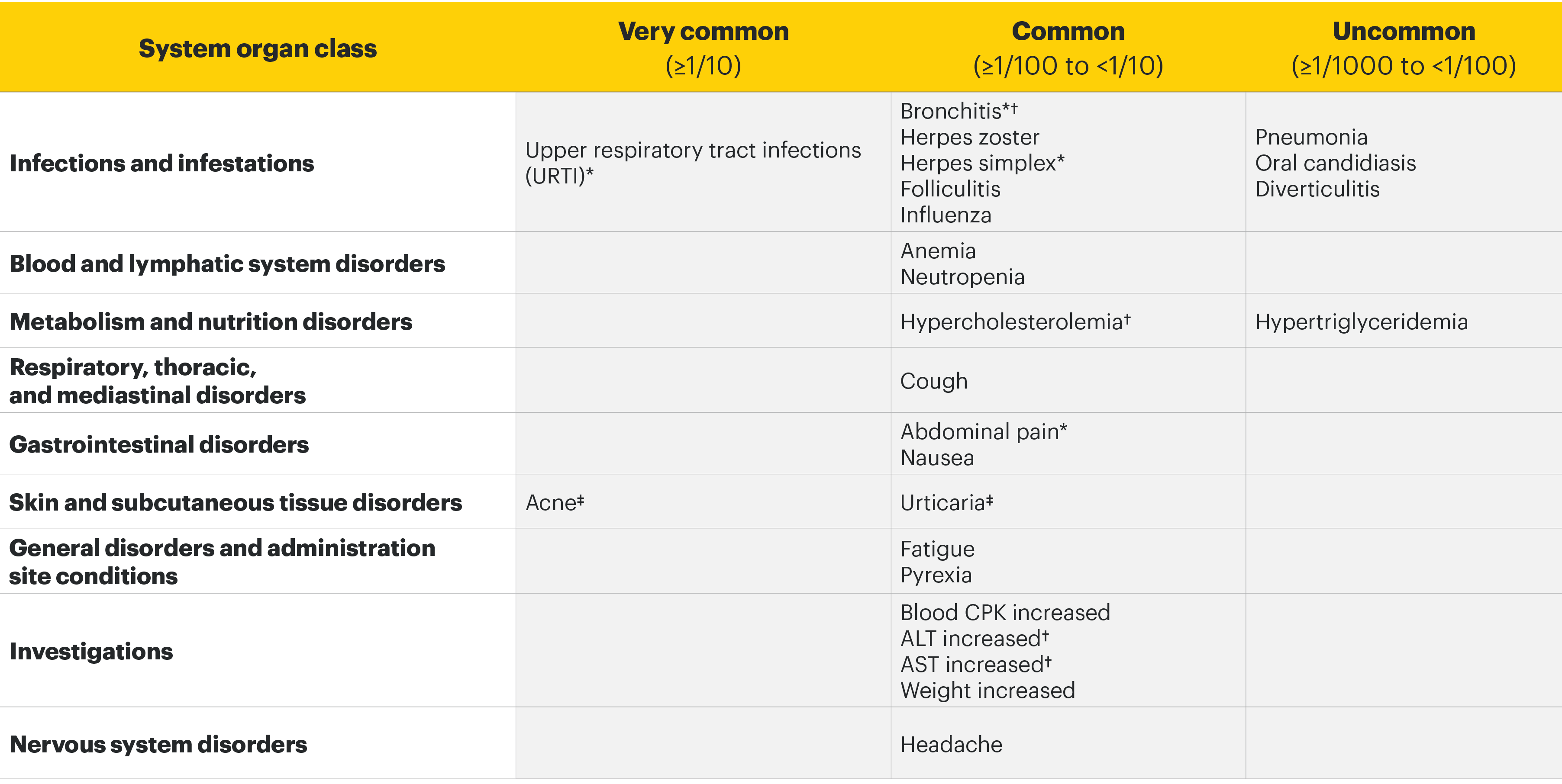
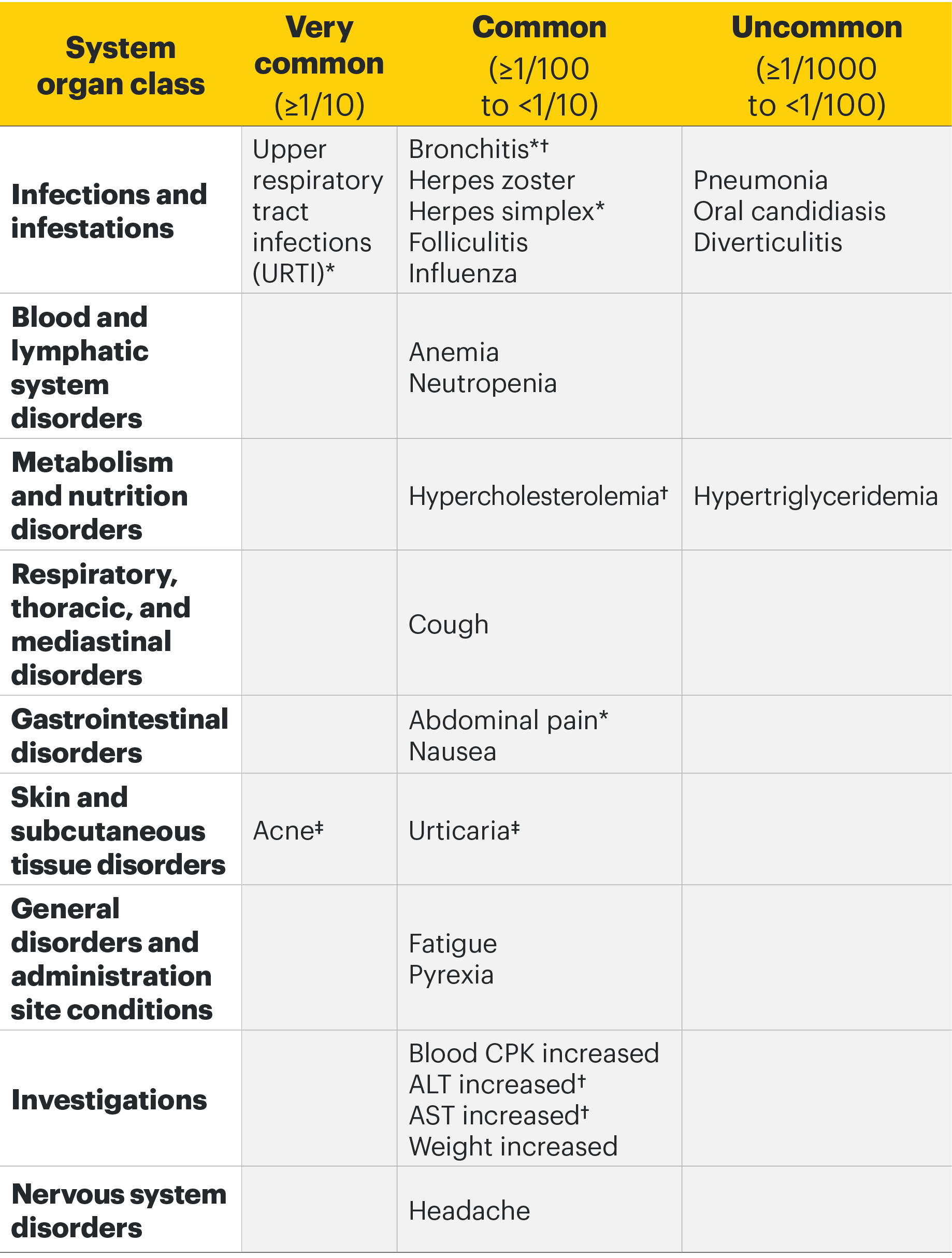
Most common adverse drug reactions1 The most commonly reported adverse drug reactions were upper respiratory tract infections, blood creatine phosphokinase (CPK) increased, alanine transaminase (ALT) increased, bronchitis, nausea, cough, aspartate transaminase (AST) increased, and hypercholesterolaemia. The most common serious adverse reactions were serious infections.
Psoriatic arthritis: A higher rate of serious infections (2.6 events per 100 patient-years and 1.3 events per 100 patient-years, respectively) and hepatic transaminase elevations (ALT elevations Grade 3 and higher rates 1.4% and 0.4%, respectively) was observed in patients treated with RINVOQ in combination with MTX therapy compared to patients treated with monotherapy.1
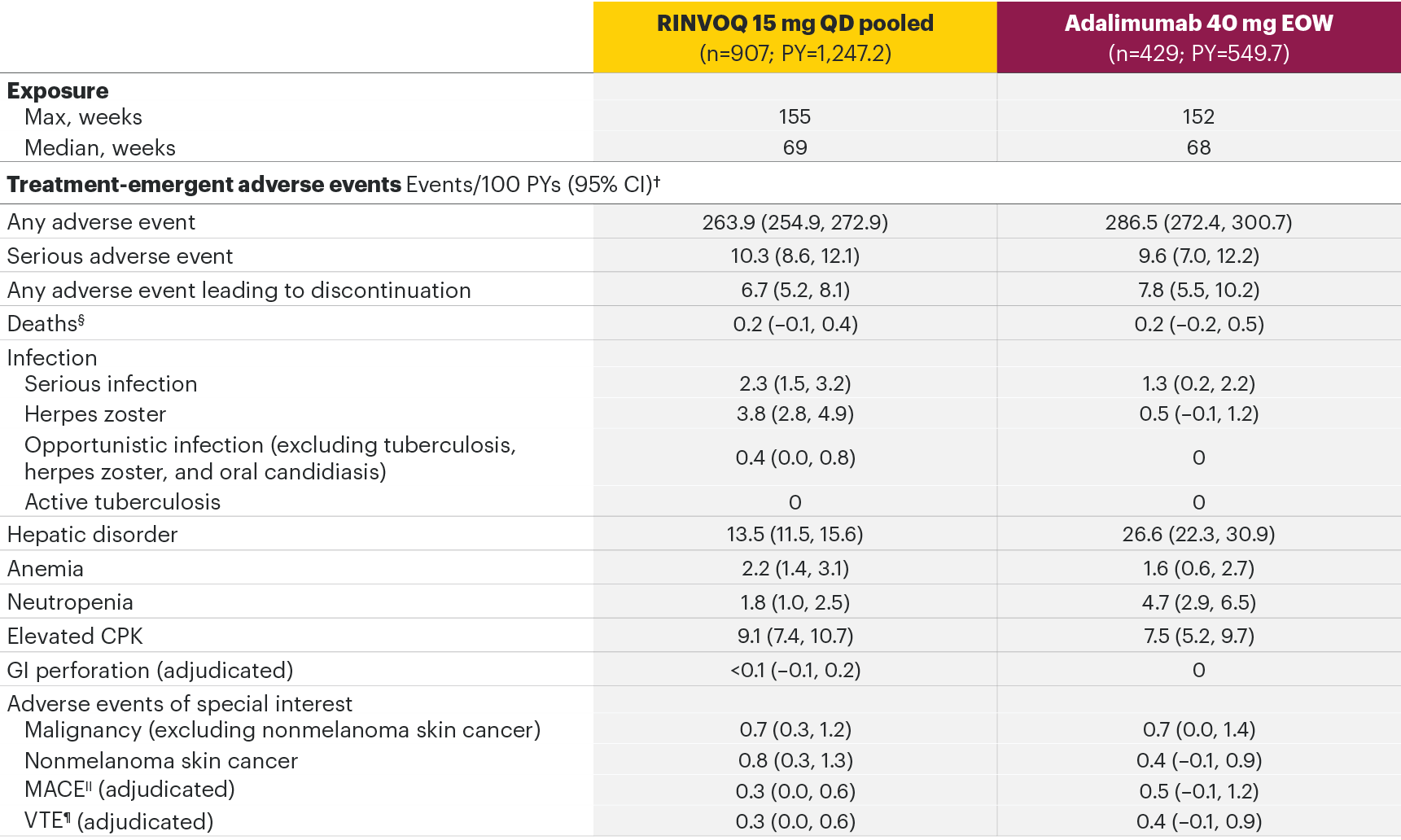
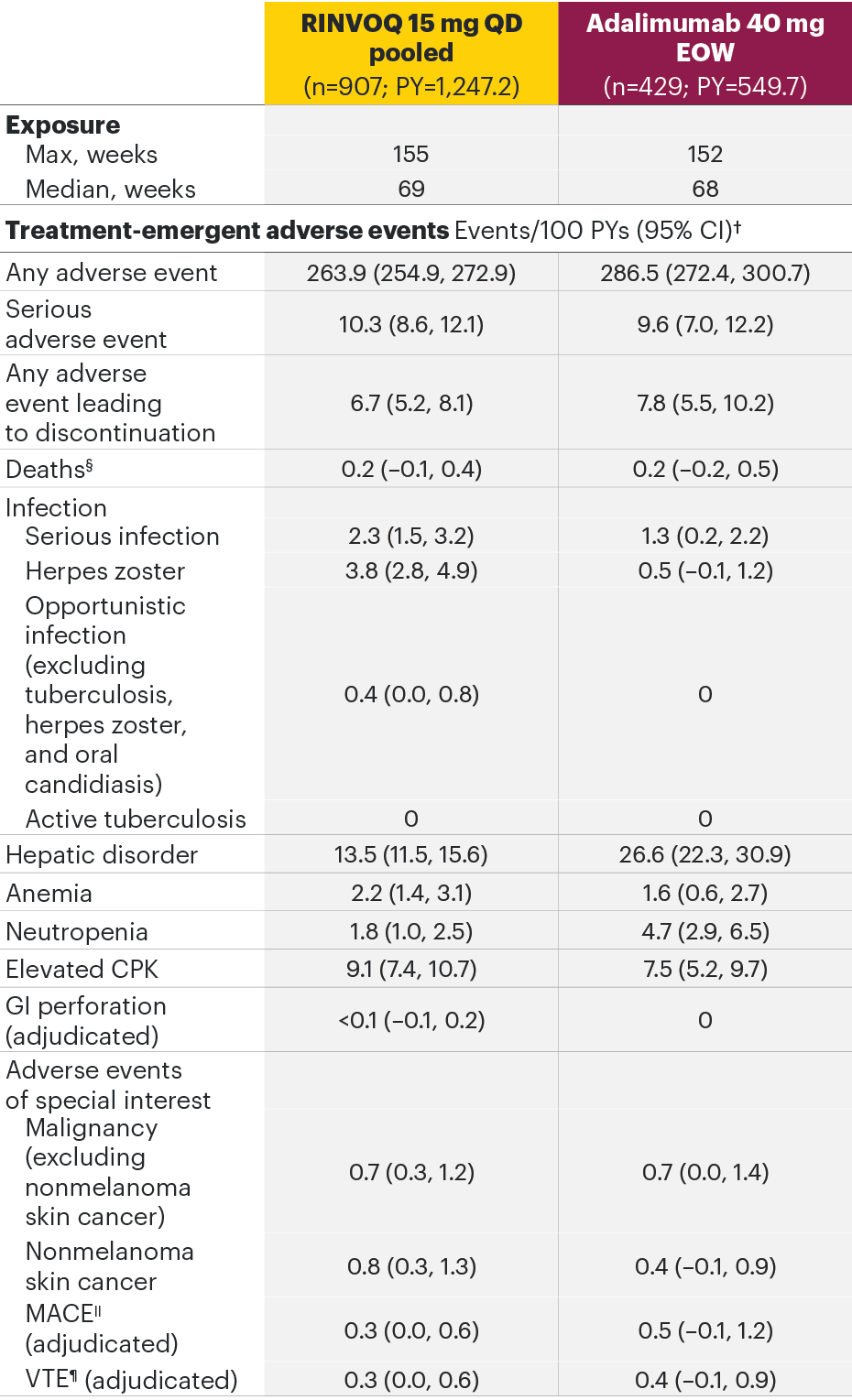
RA integrated analysisa of Phase 3 program AEs2
aSELECT-EARLY, SELECT-COMPARE, SELECT-MONOTHERAPY, SELECT-NEXT, SELECT-BEYOND, and SELECT-CHOICE.
The most common AEs for RINVOQ 15 mg QD in the RA integrated safety analysis were upper respiratory tract infection, nasopharyngitis, and urinary tract infection.
*In RA studies, patients who switched from placebo, adalimumab, MTX, or abatacept to RINVOQ were included in the RINVOQ analysis set from the start of RINVOQ, while those who switched from RINVOQ to adalimumab were included in the adalimumab analysis set from the start of adalimumab and were censored at the time of switch. MTX monotherapy was censored at time of rescue to combination therapy (addition of RINVOQ).
§Both treatment and non–treatment-emergent deaths.
||MACE defined as cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke.
¶VTE defined as deep vein thrombosis and pulmonary embolism.
PsA integrated analysisb of Phase 3 program AEs4
bSELECT-PsA 1 and SELECT-PsA 2.
The most common serious infection for RINVOQ 15 mg QD in the PsA integrated safety analysis was pneumonia.
†In PsA studies, at Week 24, all patients randomized to placebo were switched to RINVOQ 15 mg or upadacitinib 30 mg QD in a blinded manner.
§Both treatment and non–treatment-emergent deaths.
||MACE defined as cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke.
¶VTE defined as deep vein thrombosis and pulmonary embolism.
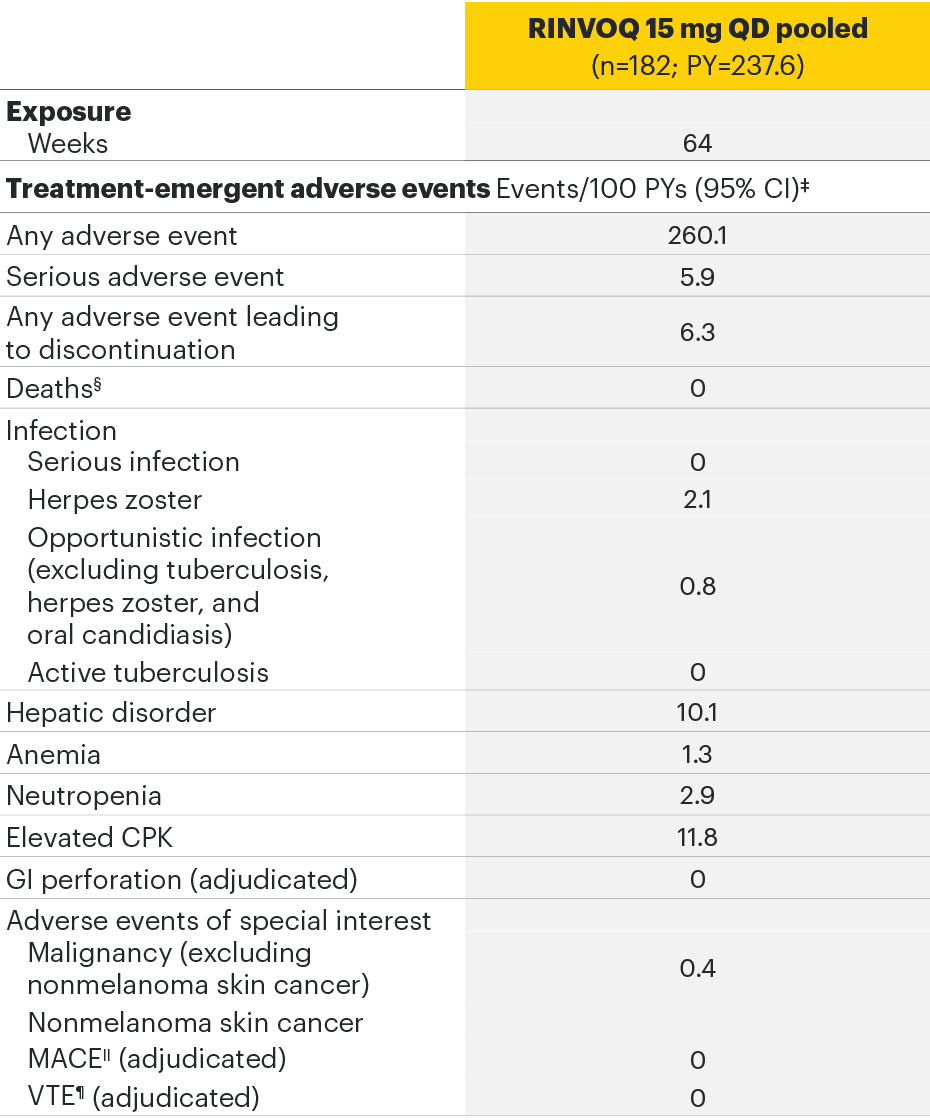
AS Phase 2/3 studyc AEs6
cSELECT-AXIS 1.
The most common AEs for RINVOQ 15 mg QD in SELECT-AXIS 1 were nasopharyngitis, increased blood creatine phosphokinase, and upper respiratory tract infection.
‡In the AS studies, at Week 14, all patients randomized to placebo were switched to RINVOQ 15 mg QD.
§Both treatment and non–treatment-emergent deaths.
||MACE defined as cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke.
¶VTE defined as deep vein thrombosis and pulmonary embolism.
Summary of the RINVOQ safety profile in RA, PsA, and AS
Contraindications1
- Hypersensitivity to the active substance or to any of the excipients
- Active tuberculosis (TB) or active serious infections
- Severe hepatic impairment
- Pregnancy
Women of childbearing potential should be advised to use effective contraception during treatment and for 4 weeks following the final dose of upadacitinib.
Most common adverse drug reactions1
In the placebo-controlled clinical trials for rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis, the most commonly reported adverse reactions (≥2% of patients in at least one of the indications with the highest rate among indications presented) with upadacitinib 15 mg were upper respiratory tract infections (19.5%), blood creatine phosphokinase (CPK) increased (8.6%), alanine transaminase increased (4.3%), bronchitis (3.9%), nausea (3.5%), cough (2.2%), aspartate transaminase increased (2.2%), and hypercholesterolaemia (2.2%).
Adverse drug reactions1
The following list of adverse reactions is based on experience from clinical studies. The frequencies in the table are based on the higher of the rates for adverse reactions reported with RINVOQ 15 mg in rheumatologic disease and atopic dermatitis clinical trials, or RINVOQ 30 mg in atopic dermatitis clinical trials. When notable differences in frequency were observed between indications, these are presented in the footnotes below the table. The 30 mg dose is not an approved dose in RA, PsA, or AS.
Psoriatic arthritis1
Overall, the safety profile observed in patients with active psoriatic arthritis treated with RINVOQ 15 mg was consistent with the safety profile observed in patients with rheumatoid arthritis. A higher rate of serious infections (2.6 events per 100 patient-years and 1.3 events per 100 patient-years, respectively) and hepatic transaminase elevations (ALT elevations Grade 3 and higher rates 1.4% and 0.4%, respectively) was observed in patients treated with RINVOQ in combination with MTX therapy compared to patients treated with monotherapy.
Ankylosing spondylitis1
Overall, the safety profile observed in patients with active ankylosing spondylitis treated with RINVOQ 15 mg was consistent with the safety profile observed in patients with rheumatoid arthritis. No new safety findings were identified.
For additional safety information, please see the Important Safety Information link at the top of the page, or for complete prescribing information, the RINVOQ SmPC.
*Presented as grouped term.
†ln atopic dermatitis trials, the frequency of bronchitis, hypercholesterolaemia, ALT increased, and AST increased was uncommon.
‡In rheumatologic disease trials, the frequency was common for acne and uncommon for urticaria.
Special Warnings and Precautions1
- Serious infections: RINVOQ should not be initiated in patients with an active, serious infection, including localized infections. Closely monitor patients for the development of signs and symptoms of infection during and after treatment with RINVOQ. If a patient develops a serious or opportunistic infection, RINVOQ should be interrupted. As there is a higher incidence of infections in the elderly ≥65 years of age, caution should be used when treating this population.
- Tuberculosis: Patients should be screened for tuberculosis (TB) before starting RINVOQ. Treatment should not be initiated in patients with active TB. Anti-TB therapy should be considered prior to initiation of RINVOQ in patients with previously untreated latent TB or in patients with risk factors for TB infection. Monitor patients for the development of signs and symptoms of TB, including patients who tested negative for latent TB infection prior to initiating therapy.
- Viral reactivation: Screening for viral hepatitis and monitoring for reactivation should be performed before starting and during treatment with RINVOQ. The risk of herpes zoster appears to be higher in Japanese patients treated with upadacitinib. If a patient develops herpes zoster, consider interruption of RINVOQ therapy until the episode resolves. If hepatitis B virus DNA is detected while receiving RINVOQ, a liver specialist should be consulted.
- Nonmelanoma skin cancer: Periodic skin examination is recommended for patients who are at increased risk for skin cancer.
- Vaccinations: Use of live, attenuated vaccines during or immediately prior to RINVOQ treatment is not recommended. Prior to initiating RINVOQ, it is recommended that patients be brought up to date with all immunizations, including prophylactic zoster vaccinations, in agreement with current immunization guidelines.
- Venous thromboembolism: RINVOQ should be used with caution in patients at high risk for deep venous thrombosis (DVT)/pulmonary embolism (PE). If clinical features of DVT/PE occur, RINVOQ treatment should be discontinued and patients should be evaluated promptly, followed by appropriate treatment.
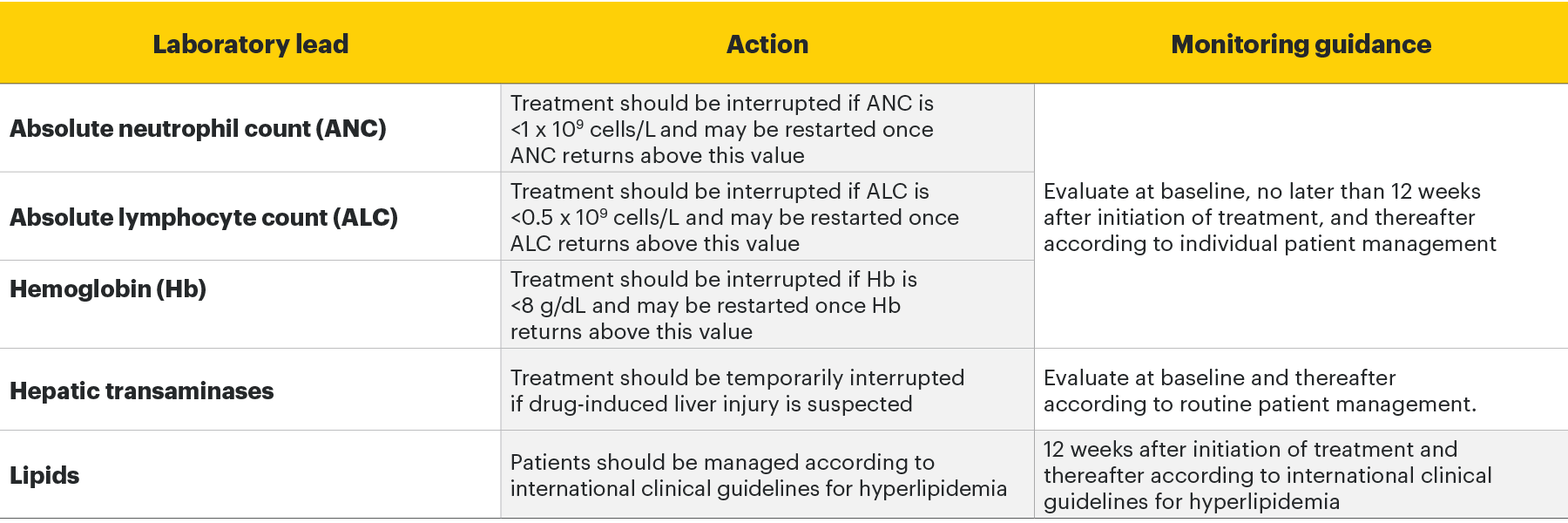
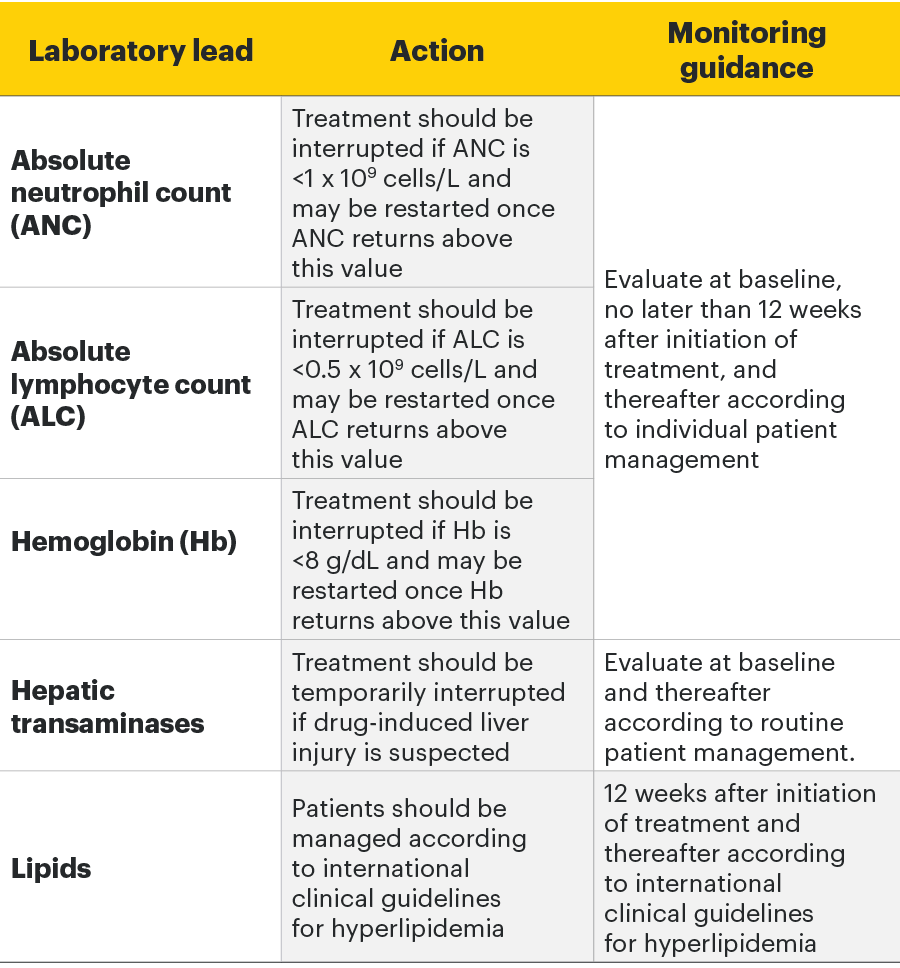
Monitoring requirements
Treatment with RINVOQ should not be initiated if:1
- Absolute neutrophil count <1 x 109 cells/L
- Absolute lymphocyte count <0.5 x 109 cells/L
- Hemoglobin <8 g/dL
Laboratory measures and monitoring guidance1
Elderly
There are limited data in patients aged 75 years and older.
Renal impairment
No dose adjustment is required in patients with mild or moderate renal impairment. There are limited data on the use of RINVOQ in subjects with severe renal impairment. RINVOQ should be used with caution in patients with severe renal impairment. The use of upadacitinib has not been studied in subjects with end-stage renal disease.
Hepatic impairment
No dose adjustment is required in patients with mild (Child-Pugh A) or moderate (Child-Pugh B) hepatic impairment. RINVOQ should not be used in patients with severe (Child-Pugh C) hepatic impairment.
Pediatric
The safety and efficacy of RINVOQ in children and adolescents with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis aged 0 to less than 18 years have not yet been established. No data are available.
RINVOQ Important Safety Information1
Contraindications
RINVOQ is contraindicated in patients hypersensitive to the active substance or to any of the excipients, in patients with active tuberculosis (TB) or active serious infections, in patients with severe hepatic impairment, and during pregnancy.
Special warnings and precautions for use
Immunosuppressive medicinal products
Use in combination with other potent immunosuppressants is not recommended.
Serious infections
Serious and sometimes fatal infections have been reported in patients receiving upadacitinib. The most frequent serious infections reported included pneumonia and cellulitis. Cases of bacterial meningitis have been reported. Among opportunistic infections, TB, multidermatomal herpes zoster, oral/esophageal candidiasis, and cryptococcosis have been reported with upadacitinib. As there is a higher incidence of infections in patients ≥65 years of age, caution should be used when treating this population.
Viral reactivation
Viral reactivation, including cases of herpes zoster, was reported in clinical studies. The risk of herpes zoster appears to be higher in Japanese patients treated with upadacitinib.
Vaccinations
The use of live, attenuated vaccines during or immediately prior to therapy is not recommended. It is recommended that patients be brought up to date with all immunizations, including prophylactic zoster vaccinations, prior to initiating upadacitinib, in agreement with current immunization guidelines.
Malignancy
The risk of malignancies, including lymphoma is increased in patients with rheumatoid arthritis (RA). Malignancies, including nonmelanoma skin cancer (NMSC), have been reported in patients treated with upadacitinib. Consider the risks and benefits of upadacitinib treatment prior to initiating therapy in patients with a known malignancy other than a successfully treated NMSC or when considering continuing upadacitinib therapy in patients who develop a malignancy.
Hematological abnormalities
Treatment should not be initiated, or should be temporarily interrupted, in patients with hematological abnormalities observed during routine patient management.
Diverticulitis
Upadacitinib should be used with caution in patients with diverticular disease and especially in patients chronically treated with concomitant medications associated with an increased risk of diverticulitis.
Cardiovascular risk
RA patients have an increased risk for cardiovascular disorders. Patients treated with upadacitinib should have risk factors (e.g., hypertension, hyperlipidemia) managed as part of usual standard of care.
Lipids
Upadacitinib treatment was associated with dose-dependent increases in lipid parameters, including total cholesterol, low-density lipoprotein cholesterol, and high-density lipoprotein cholesterol.
Hepatic transaminase elevations
Treatment with upadacitinib was associated with an increased incidence of liver enzyme elevation compared to placebo.
Venous thromboembolisms
Events of deep vein thrombosis (DVT) and pulmonary embolism (PE) have been reported in patients receiving JAK inhibitors, including upadacitinib. Upadacitinib should be used with caution in patients at high risk for DVT/PE.
Adverse reactions
The most commonly reported adverse reactions in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis clinical trials (≥2% of patients in at least one of the indications) with upadacitinib 15 mg were upper respiratory tract infections, blood creatine phosphokinase (CPK) increased, alanine transaminase increased (ALT), bronchitis, nausea, cough, aspartate transaminase increased (AST), and hypercholesterolemia. The most common serious adverse reactions were serious infections.
The safety profile of upadacitinib with long term treatment was generally similar to the safety profile during the placebo-controlled period across indications.
Overall, the safety profile observed in patients with psoriatic arthritis or active ankylosing spondylitis treated with upadacitinib 15 mg was consistent with the safety profile observed in patients with RA.
This is not a complete summary of all safety information.
Please see the RINVOQ Summary of Product Characteristics for complete prescribing information.
- RINVOQ [Summary of Product Characteristics]. AbbVie Deutschland GmbH & Co. KG.
- Cohen SB, van Vollenhoven R, Curtis JR, et al. Integrated safety profile of upadacitinib with up to 4.5 years of exposure in patients with rheumatoid arthritis [EULAR abstract POS0220]. Ann Rheum Dis. 2021;80(suppl 1):328-329.
- Cohen SB, van Vollenhoven R, Curtis JR, et al. Integrated safety profile of upadacitinib with up to 4.5 years of exposure in patients with rheumatoid arthritis. Poster presented at: EULAR 2021 Virtual Congress; June 2–5, 2021.
- Burmester GR, Winthrop K, Blanco R, et al. Safety profile of upadacitinib up to 3 years in patients with psoriatic arthritis: an integrated analysis from the Phase 3 program. [EULAR abstract AB0522]. Ann Rheum Dis. 2021;80(suppl 1):1287-1288.
- Burmester GR, Winthrop K, Nash P, et al. Safety profile of upadacitinib in psoriatic arthritis: integrated analysis from two Phase 3 trials. Poster presented at: EULAR 2020 E-Congress; June 3–6, 2020.
- Deodhar A, van der Heijde D, Sieper J, et al. Upadacitinib in active ankylosing spondylitis: 1-year results from the double-blind, placebo-controlled SELECT-AXIS 1 study and open-label extension [published online July 1, 2021]. Arthritis Rheumatol. doi:10.1002/art.41911