WELL-STUDIED SAFETY PROFILE THROUGH 12 WEEKS2
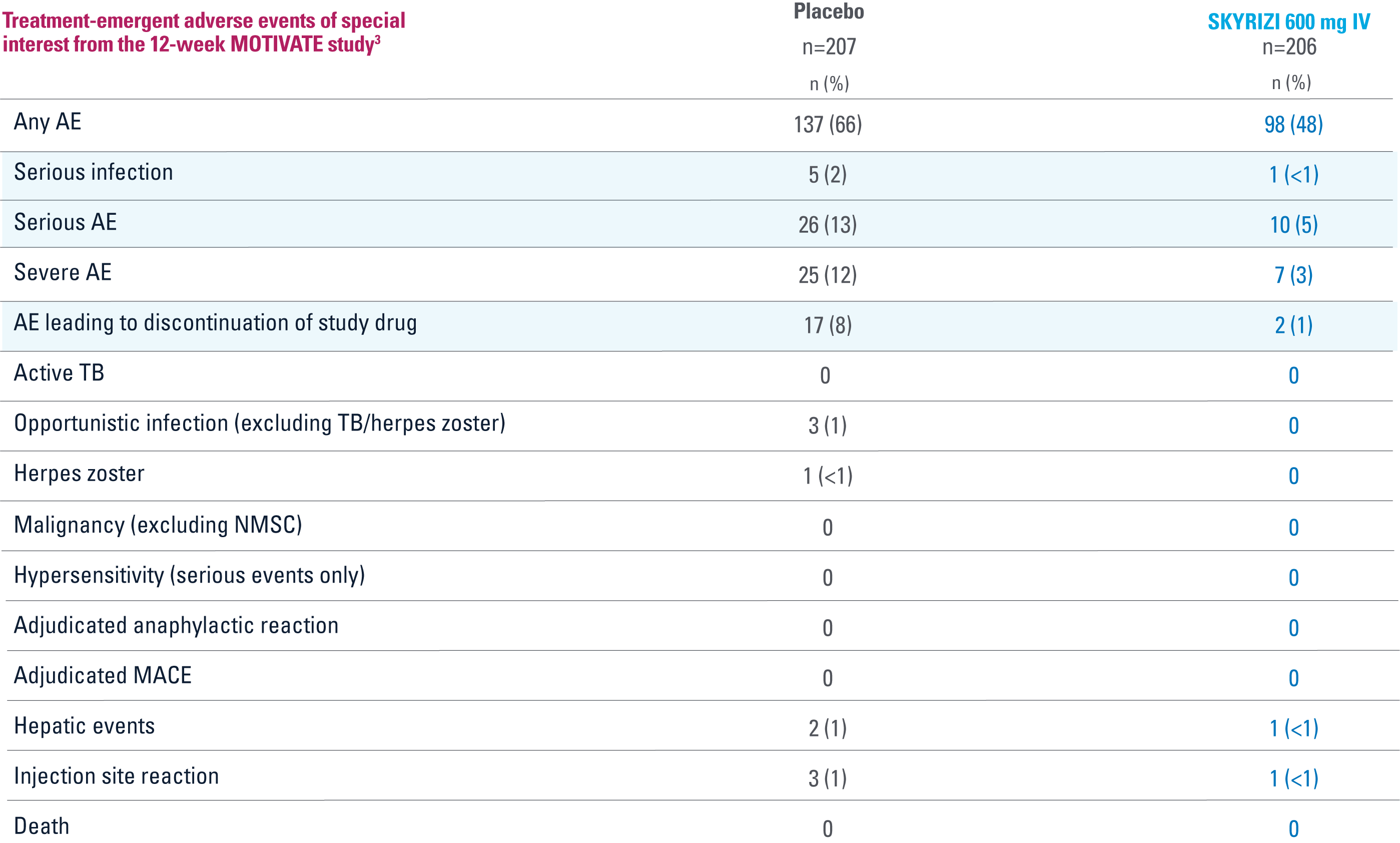
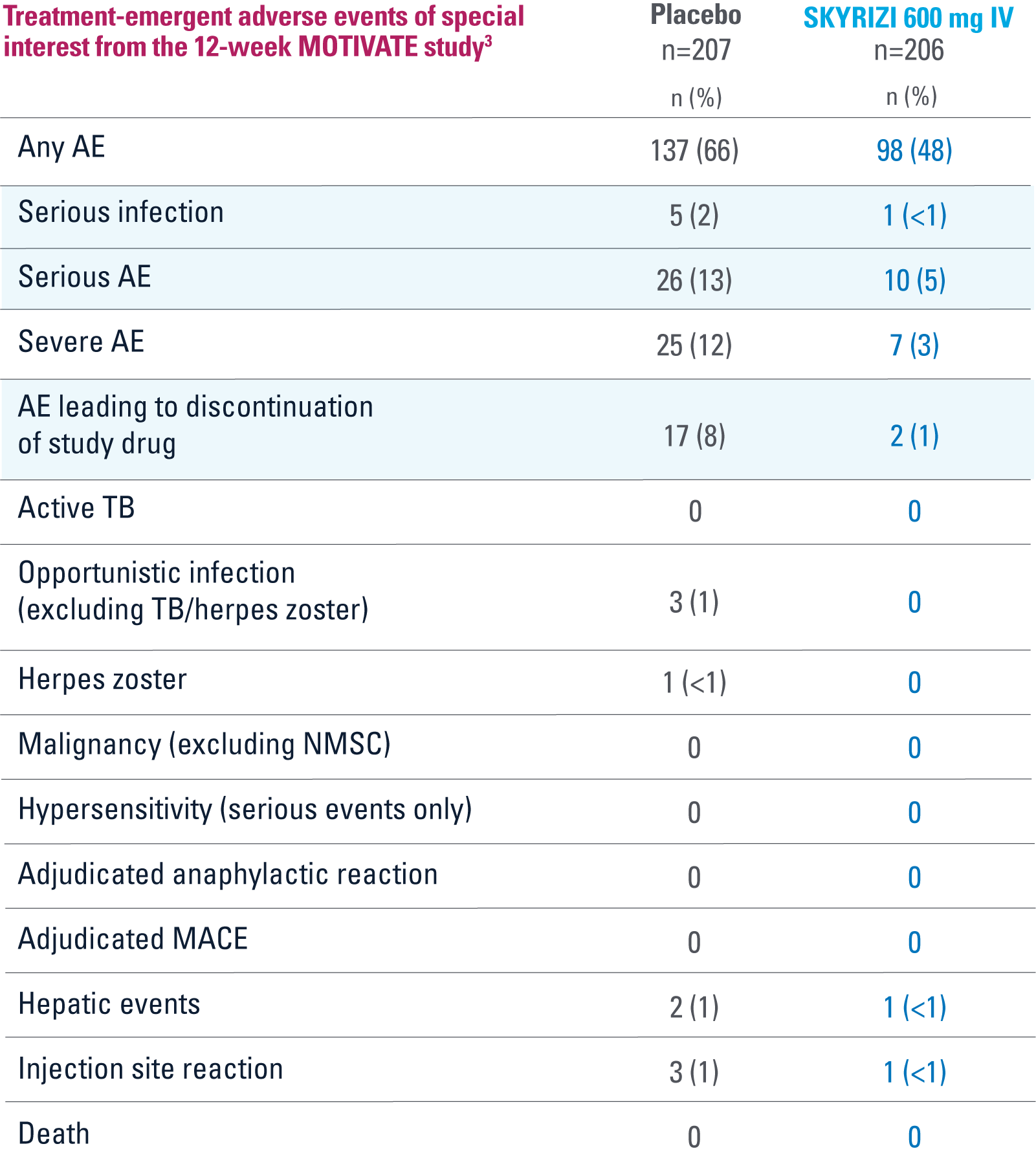
MOTIVATE
OVERALL, THE SAFETY PROFILE OBSERVED IN PATIENTS WITH CROHN’S DISEASE TREATED WITH RISANKIZUMAB WAS CONSISTENT WITH THE SAFETY PROFILE OBSERVED IN PATIENTS WITH PLAQUE PSORIASIS
WELL-STUDIED SAFETY PROFILE THROUGH 12 WEEKS2
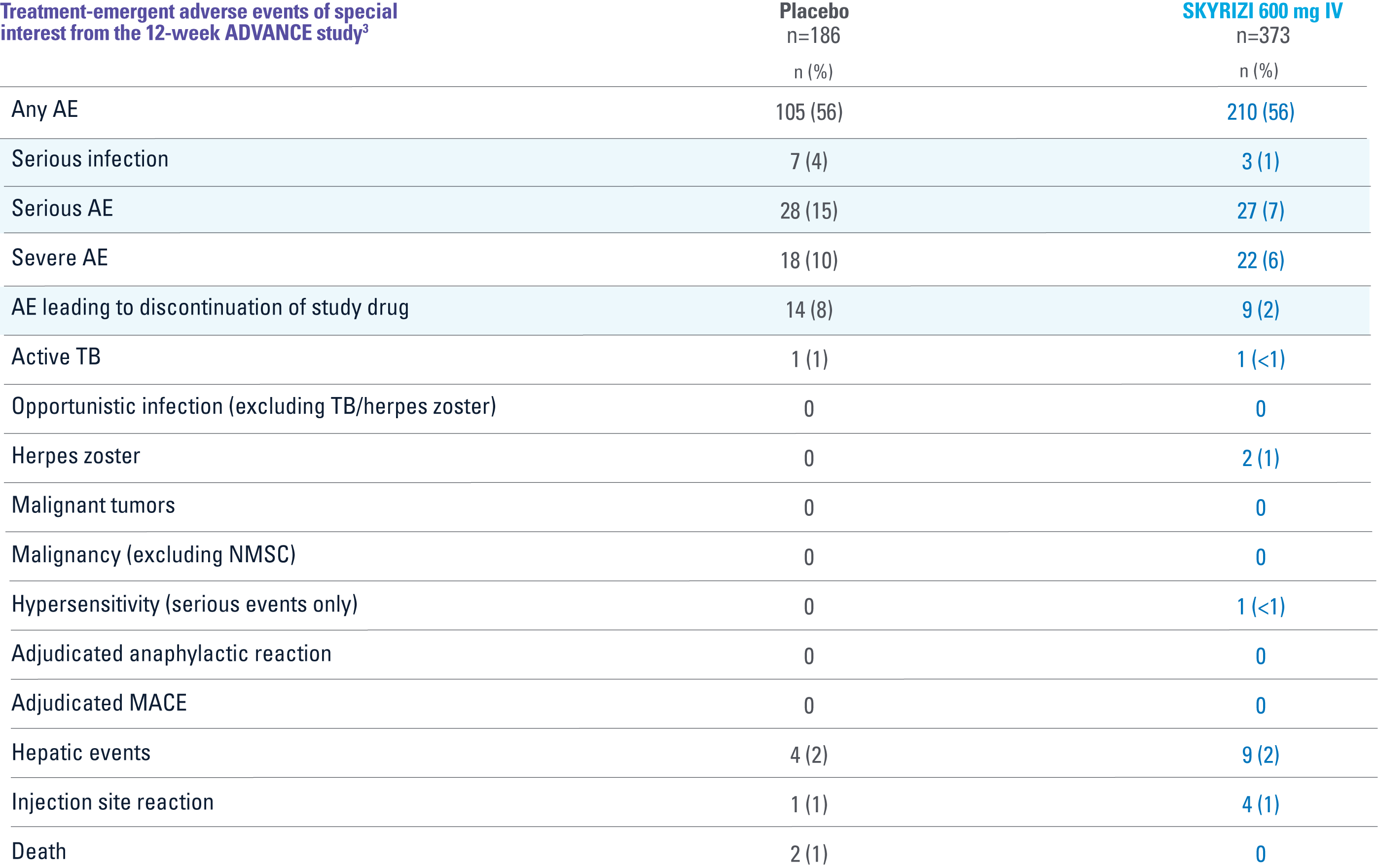
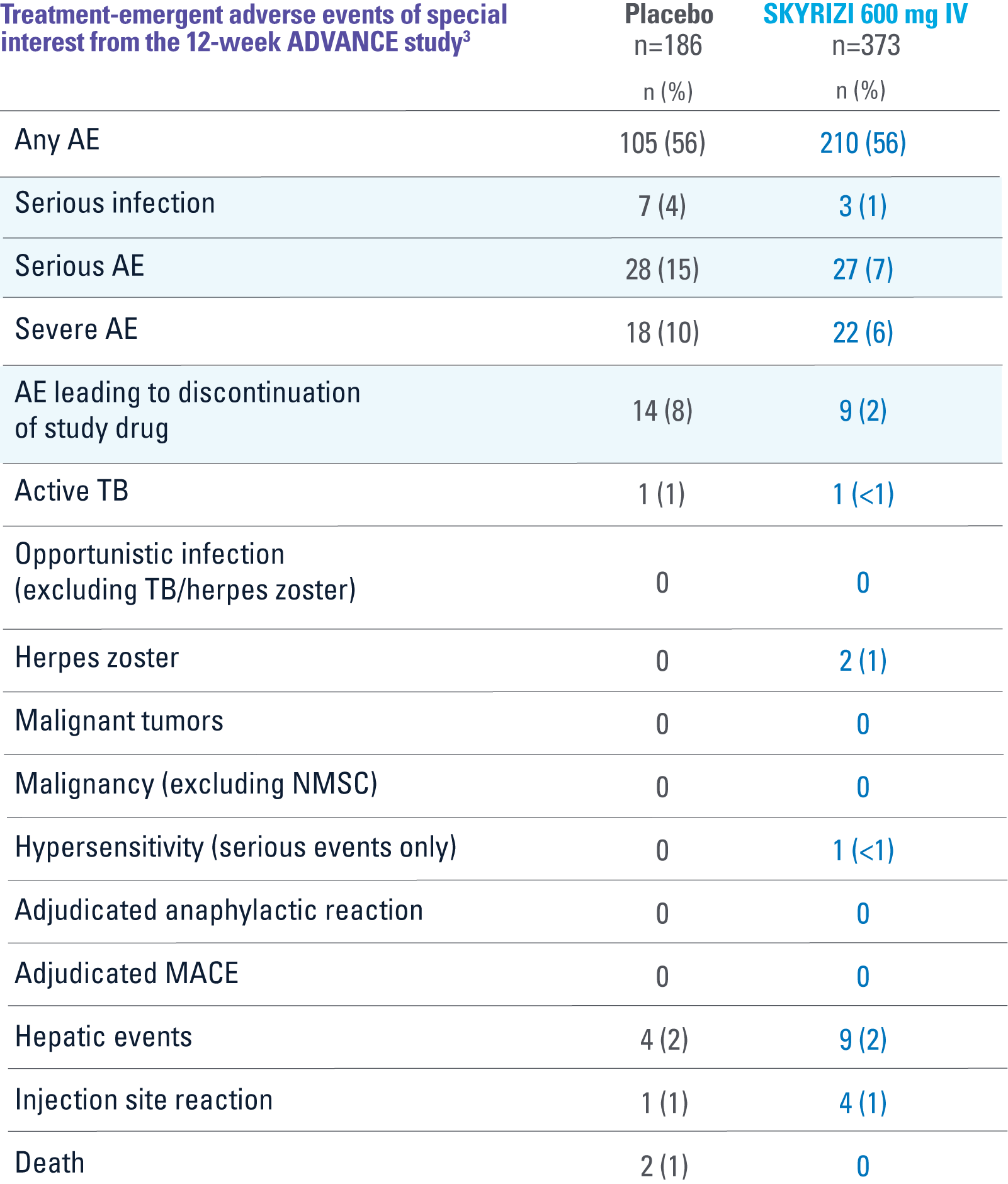
ADVANCE
OVERALL, THE SAFETY PROFILE OBSERVED IN PATIENTS WITH CROHN’S DISEASE TREATED WITH RISANKIZUMAB WAS CONSISTENT WITH THE SAFETY PROFILE OBSERVED IN PATIENTS WITH PLAQUE PSORIASIS
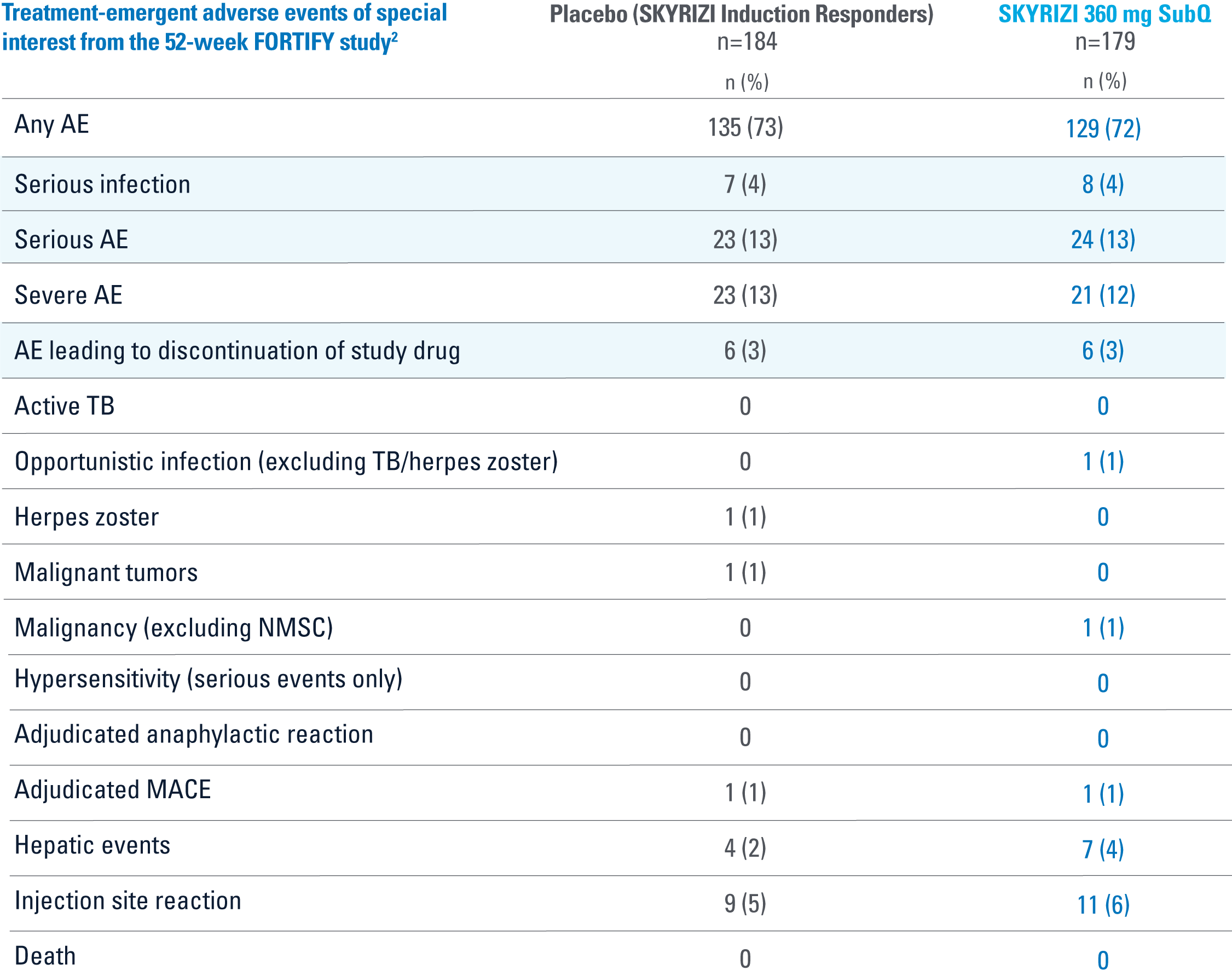
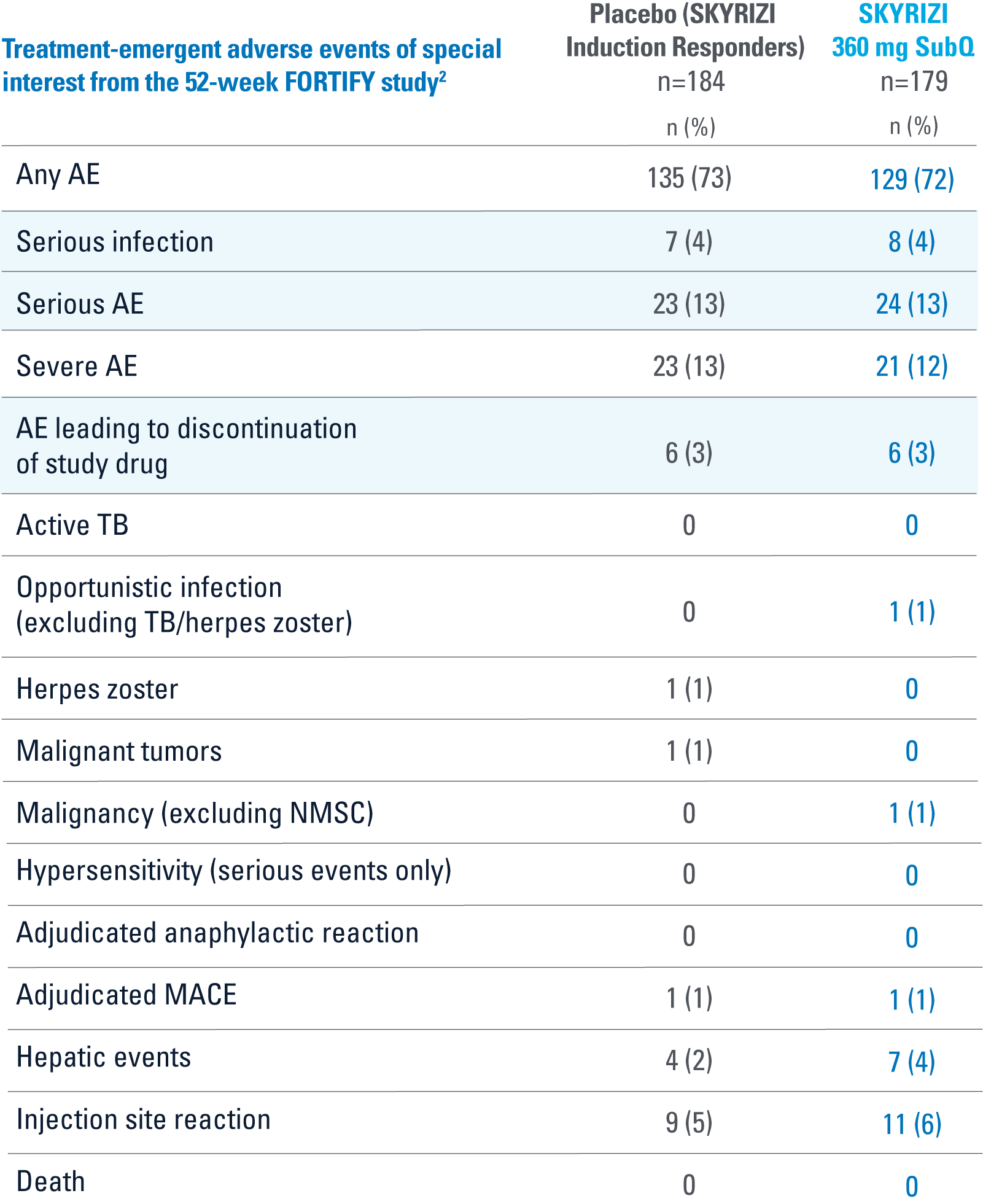
WELL-STUDIED SAFETY PROFILE THROUGH 52 WEEKS3
FORTIFY
OVERALL, THE SAFETY PROFILE OBSERVED IN PATIENTS WITH CROHN’S DISEASE TREATED WITH RISANKIZUMAB WAS CONSISTENT WITH THE SAFETY PROFILE OBSERVED IN PATIENTS WITH PLAQUE PSORIASIS
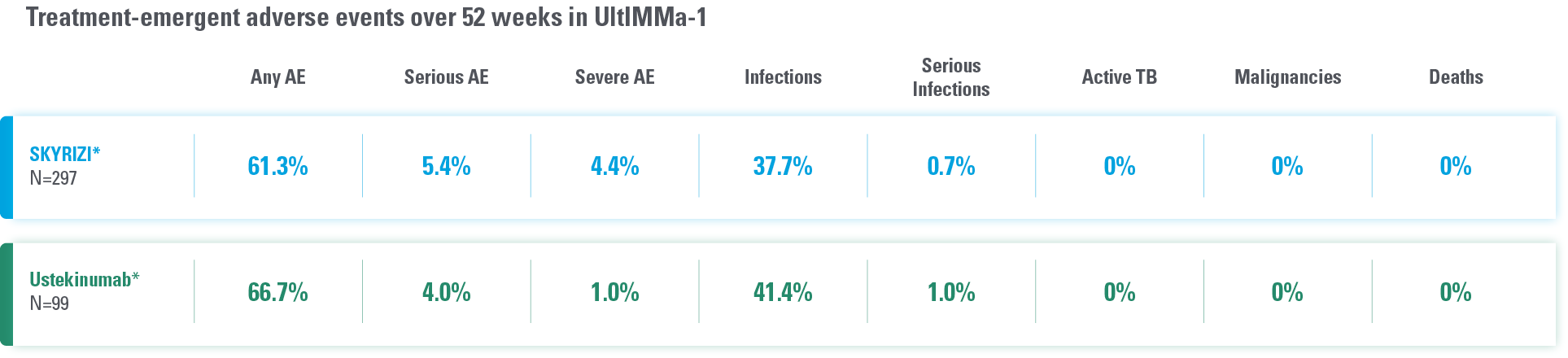
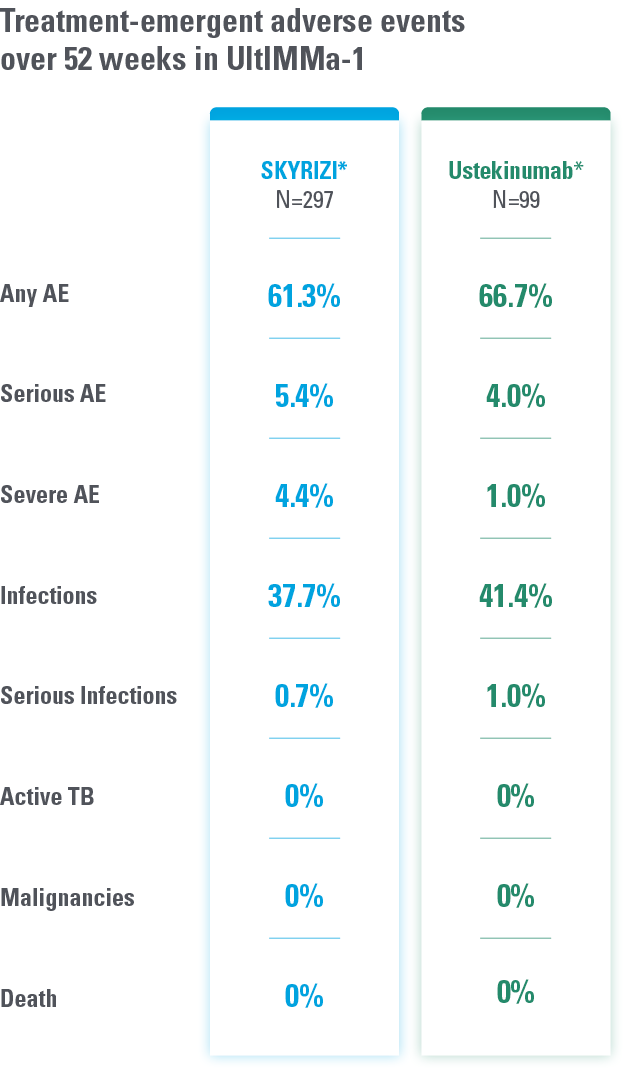
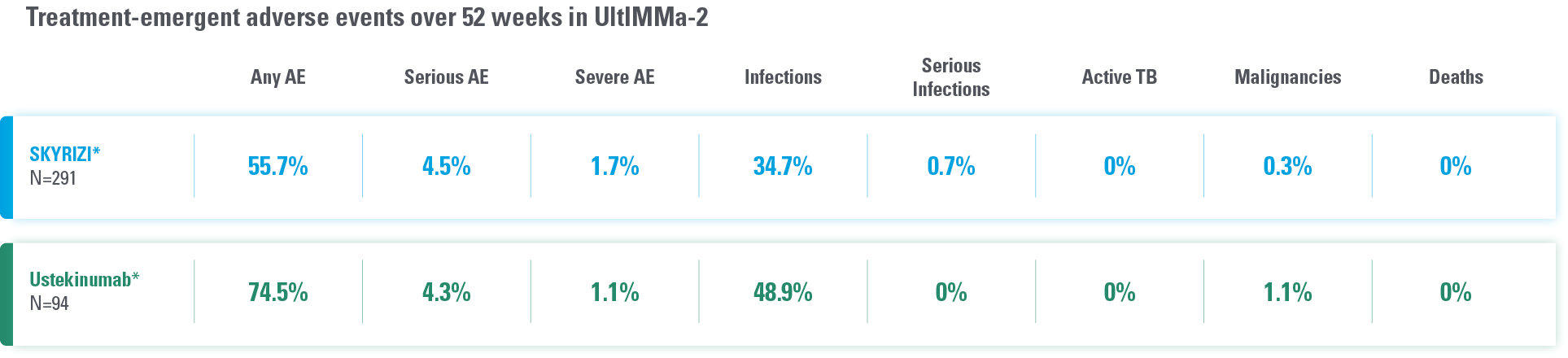
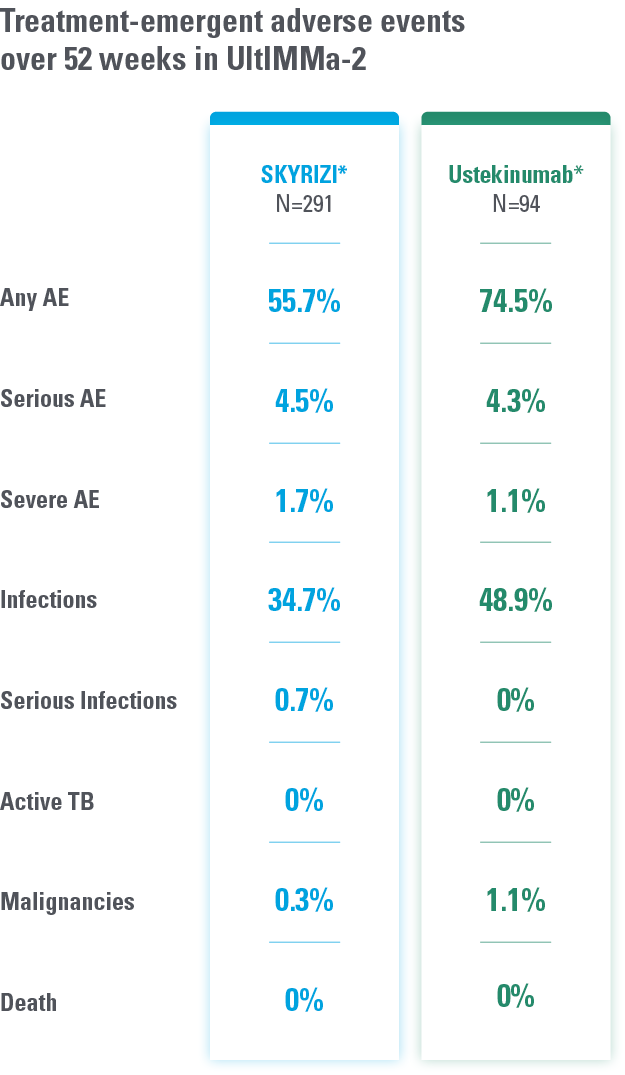
DATA FROM 2 PIVOTAL PLAQUE PSORIASIS TRIALS AT WEEK 524
Overall safety profile of SKYRIZI was similar to that of the ustekinumab treatment groups throughout the duration of both studies.
OVERALL, THE SAFETY PROFILE OBSERVED IN PATIENTS WITH CROHN’S DISEASE TREATED WITH RISANKIZUMAB WAS CONSISTENT WITH THE SAFETY PROFILE OBSERVED IN PATIENTS WITH PLAQUE PSORIASIS
*Study Design: UltIMMa-1 (N=506) and UltIMMa-2 (N=491) were replicate phase 3, randomized, double-blind, placebo- and active-controlled studies to evaluate the efficacy and safety of SKYRIZI (150 mg) vs placebo over 16 weeks and ustekinumab (45 mg or 90 mg, based on screening weight) over 52 weeks in adult patients with moderate to severe plaque psoriasis.
Safety analyses were performed using a safety analysis set (all patients who received ≥1 dose of study drug).
AE=adverse event; IV=intravenous; MACE=major adverse cardiac event; NMSC=nonmelanoma skin cancer; SubQ=subcutaneous; TB=tuberculosis.
Indication1
SKYRIZI is indicated for the treatment of adult patients with moderately to severely active Crohnʼs disease who have had an inadequate response to, lost response to, or were intolerant to conventional therapy or a biologic therapy.
Important Safety Information1
Affiliate to insert local ISI.
References: 1. SKYRIZI [Summary of Product Characteristics]. Ludwigshafen, Germany: AbbVie Deutschland GmbH & Co; January 2024. 2. D’Haens G, Panaccione R, Baert F, et al. Risankizumab as induction therapy for Crohn’s disease: results from the phase 3 ADVANCE and MOTIVATE induction trials. Lancet. 2022;399(10340):2015-2030. doi:10.1016/S0140-6736(22)00467-6 3. Ferrante M, Panaccione R, Baert F, et al. Risankizumab as maintenance therapy for moderately to severely active Crohn’s disease: results from the multicentre, randomised, double-blind, placebo-controlled, withdrawal phase 3 FORTIFY maintenance trial. Lancet. 2022;399(10340):2031-2046. doi:10.1016/S0140-6736(22)00466-4 4. Gordon KB, Strober B, Lebwohl M, et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet. 2018;392(10148):650-661. doi:10.1016/S0140-6736(18)31713-6